Baby formula supply should return to normal in two months, FDA commissioner says
FDA Commissioner Dr. Robert Califf said it will take about two months for shelves to be full with formula again.

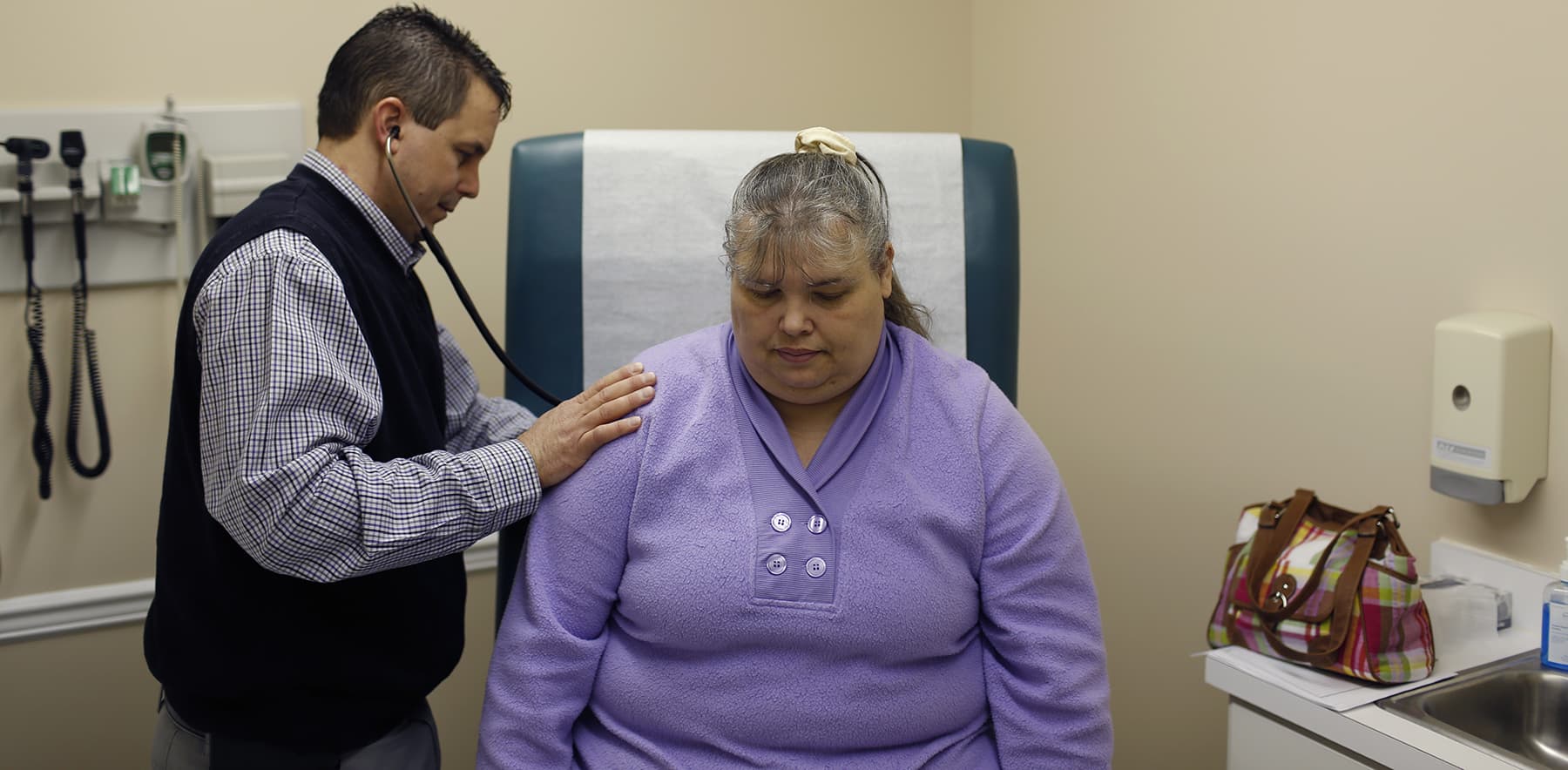
Shelves normally meant for baby formula sit nearly empty at a store in downtown Washington, DC, on May 22, 2022.
Samuel Corum | AFP | Getty Images
Food and Drug Administration Commissioner Dr. Robert Califf told lawmakers on Thursday that it will take until July before store shelves across the country are filled with baby formula again.
"It's going to be gradual improvement up to probably somewhere around two months until the shelves are replete again," Califf said during testimony before the Senate Health, Education, Labor and Pensions Committee.
Abbott plans to resume production at its plant in Sturgis, Michigan, on June 4, and it will start shipping out its specialty formula EleCare on June 20. The company has previously said it would take six to eight weeks for its formula to arrive in stores once production restarts.
The FDA has eased import restrictions to allow foreign manufacturers to send formula to the U.S., and the Defense Department is airlifting in the equivalent of 1.5 million bottles of formula from Europe.
Abbott closed the plant and recalled several infant formula products in February after FDA inspectors found Cronobacter bacteria at the facility. The plant closure and recall triggered a nationwide baby formula shortage, forcing some parents to drive for hours to find food for their infants.
Four manufacturers — Abbott, Mead Johnson Nutrition, Nestle USA and Perrigo — control 90% of the domestic infant formula market in the U.S. Abbott alone has a 40% share of the U.S. baby formula market. The Michigan facility is responsible for 40% of the company's U.S. production.
Baby formula became increasingly scarce last year as the Covid pandemic disrupted supply chains and families stocked up on formula. However, spot shortages in some parts of the country turned into a national crisis about a month ago as parents started stocking up again on formula in response to the Abbott recall, Califf said.
"We knew that ceasing plant operations would create supply problems, but we had no choice given the insanitary conditions," Califf told lawmakers.
FDA inspectors found shocking conditions inside the plant, including bacteria growing from multiple sites, standing water, roof leaks and inadequate hygiene, he said.
Four infants who consumed formula from the plant fell ill with Cronobacter infections, and two of them died. However, the FDA and the Centers for Disease Control and Prevention have not found a link between the infant illnesses and the bacteria found at the plant, Califf said.
Still, the plant cannot reopen until Abbott takes hundreds of steps to fulfill the requirements under a federal consent decree to come into compliance with U.S. food safety standards.
"You can't just open a plant with bacteria growing in it," Califf said.
"Would you go in a kitchen next door if there was bacteria growing all over the place and standing water and people tromping through with mud on their feet?" he asked lawmakers.
Senators slammed the FDA for taking too long to physically inspect the plant after receiving reports of contamination at the facility. The FDA first received a report in September that an infant who consumed powdered formula from the Michigan plant fell ill with a Cronobacter infection.
A whistleblower sent a complaint to the FDA in October about lax practices and regulatory violations at the plant. However, the FDA did not actually inspect the plant until late January.
Califf acknowledged that the FDA responded too slowly to the whistleblower complaint and took too long to conduct an inspection. Though the FDA received the whistleblower complaint in October, it was not escalated up the chain of command to the agency's leadership until February.
"There [are] systemic issues at FDA and in our interactions with the industry and in our authorities that need to be corrected," Califf told lawmakers.

 BigThink
BigThink