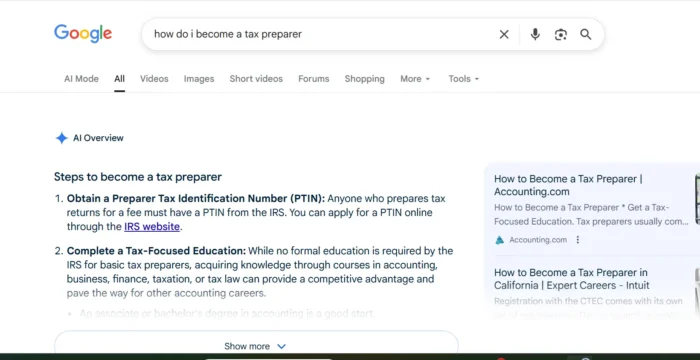
FDA advisors recommend GSK's RSV vaccine for older adults, but flag potential safety risks
A majority of the FDA panel said GSK's vaccine safety data was adequate, and the advisors were unanimous that the shot's efficacy was good.

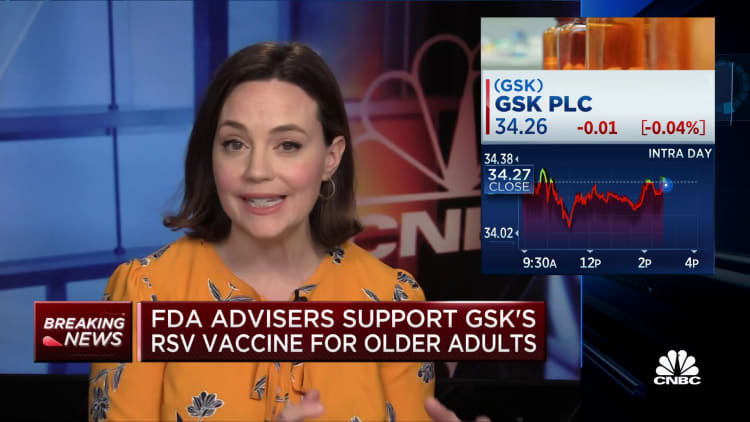
The Food and Drug Administration's independent panel of advisors on Wednesday recommended GlaxoSmithKline's RSV vaccine for adults ages 60 and older, though they flagged potential safety issues over nervous system disorders that may be tied to the shot.
Ten of the FDA advisors said the safety data on GSK's vaccine was adequate, while two said it was not. The committee unanimously said the vaccine efficacy data was sufficient.
The panel reached a similar conclusion in a narrow 7 to 4 vote Tuesday on Pfizer's application to clear its RSV vaccine. While the advisors erred toward recommending approval, they also raised concern over a possible link to Guillain-Barre syndrome. One scientist abstained from that vote.
Respiratory syncytial virus kills thousands of seniors every year. There currently is no approved vaccine for RSV. GSK's shot is administered as a single 120 microgram dose.
Both companies have asked the FDA to approve their RSV shot for adults ages 60 and older. The agency is expected to make its decision on GSK's vaccine by May 3 with Pfizer's answer expected to come that month as well. GSK's and Pfizer's respective vaccines stand to become the world's first approved vaccines to prevent the virus.
GSK's vaccine was about 83% effective at preventing lower respiratory tract disease caused by RSV during its trial, according to an FDA review of the company's data. Disease was defined as two more symptoms including shortness of breath, wheezing, cough, increased mucus production, crackles, low oxygen saturation or need of oxygen supplementation.
GSK did not have data on how long protection from the vaccine lasts and how it performs in people with weak immune systems, according to FDA.
"These data are robust and demonstrate potentially very high effectiveness against lower respiratory tract disease," said Dr. Amanda Cohn, a committee member and chief medical officer at the National Center for Immunizations and Respiratory Diseases.
But the advisors discussed at length risks of rare nervous system disorders that are possibly related to the vaccine. GSK said it is closely monitoring safety concerns during the trials and will continue to do so after a potential approval.
Dr. Hana El Sahly, the committee chair, said more safety data is needed before approval. There was a case of Guillain-Barre in the trial, and two people developed another rare nervous system disorder after receiving both the RSV and flu vaccine, one of whom died. Dr. Marie Griffin, who also sits on the panel, agreed that more data is needed.
"I just don't see why the rush on getting this vaccine approved now," said Griffin, a physician at Vanderbilt University Medical Center.
Guillain-Barre case
A 78-year-old woman in Japan was diagnosed with Guillain-Barre syndrome nine days after receiving GSK's vaccine. She was discharged from the hospital six months after vaccination. The woman was the only case of Guillain-Barre syndrome out 15,000 people who received the shot.
GSK has said there wasn't sufficient evidence to confirm a diagnosis. The FDA considers the case to be related to the vaccine.
Guillain-Barre syndrome is a rare neurological disorder with symptoms ranging from weakness to paralysis. Most people recover even from severe cases, according to the National Institutes of Health.
There were two cases of Guillain-Barre syndrome diagnosed during Pfizer's RSV vaccine trials. Griffin said the fact that such a rare disorder occurred in both companies' trials is troubling.
You can hear more from GSK CEO Emma Walmsley at CNBC's Healthy Returns on March 29th. She'll discuss the company's path moving forward with the RSV vaccine and the other medications it has in the pipeline. Learn more and register today: http://bit.ly/3DUNbRo
Griffin noted that Johnson & Johnson recorded one case of Guillain-Barre during its Covid vaccine trial. The FDA ultimately issued a warning for J&J's shot after finding an increased rate of the disease. Large clinical trials of Pfizer's and Moderna's Covid vaccines did not have any Guillain-Barre cases, she said.
"It's not something that you routinely see one or two cases," said Griffin, an FDA committee member, who voted no on the shot's safety but yes on its efficacy.
Dr. Nicholas Geagan, an FDA official, agreed that the Guillain-Barre cases in the GSK and Pfizer trials were troubling. GSK has agreed to expedited reporting of cases of the disease, Geagan said.
"It does seem concerning to have observed these cases in the context of clinical development program," Geagan told the committee. "So we are discussing with the sponsor as far as further development of subsequent safety analyses of GBS."
The FDA, in a briefing document, said the rate of Guillain-Barre syndrome in older adults is about 1 in 100,00 among people 60 years and older. In GSK's trial, it was more like 1 in 15,000.
Dr. Ann Falsey, a professor of Medicine at the University of Rochester, told the panel that the rate of Guillain-Barre system increases with age and there are other studies that place the rate at 8 to 12 per 100,000 for people ages 65 and older. Falsey participated in GSK's presentation to the committee.
Dr. Peggy Webster, head of vaccine safety at GSK, said the rate of Guillain-Barre syndrome is higher in Japan, where the trial participant who developed the condition lives.
Death during trial with RSV and flu shots
There were also two cases of another nervous system disorder, including one death, during a GSK trial in which the RSV and flu vaccines were administered together. The patients developed something called acute disseminated encephalomyelitis, a sudden attack of inflammation in the brain and spinal chord. These were the only cases of the disorder among 15,000 vaccine recipients.
The FDA said the cases are possibly related to either GSK's RSV vaccine or the flu shot that was administered with it.
A 71-year-old man developed the neurological disorder seven days after receiving the RSV and flu vaccines. He was hospitalized after being found lying on the floor shaking and shivering. He died 22 days after receiving the shots.
A woman of the same age suffered headaches with double vision, forgetfulness, shaking hands and uncoordinated movements. She showed improvement but her symptoms hadn't completely resolved as of the last update, according to the FDA.
El Sahly, the FDA committee chair, said the rate of this neurological disorder is typically .1 in 100,00 patients, primarily among children.
"So two cases in elders three to four weeks post vaccine is highly anomalous from a statistical standpoint," said El Sahly, who voted no on safety but yes on efficacy.
Adam Berger, an official at the National Institutes of Health, said he views the acute disseminated encephalomyelitis cases as likely related to co-administration of the RSV and flu shots rather than an issue with GSK's vaccine.
"I suggest heavy reliance on the post-marketing surveillance and not only just reliance but making sure there's an enforcement around the requirement for actually conducting these," said Berger, who is an FDA committee member.
There were also two cases of Bell's Palsy, which is weakness or paralysis on one side of the face. There was also a case of Grave's disease or overproduction of thyroid hormones, a case of gout, and a case of a skin condition called psoriasis.
An FDA staff report said the cases were possibly related to the vaccine.
In adults ages 65 and older, RSV causes 6,000 to 10,000 deaths and 60,000 to 160,000 hospitalizations per year, according to the Centers for Disease Control Prevention. The risk of hospitalization increases with age, and adults ages 70 and older are more vulnerable.
Among adults of all ages hospitalized with RSV, 19% require intensive care and 4% die, according to CDC data from three seasons. Mortality is the highest among seniors.
GSK said the benefit the vaccine would provide in preventing disease from RSV would outweigh any potential risks.
"Our obligation is to do what's right for the public," said Dr. David Kim, an officer in the U.S. public health service and an FDA committee member. "And in this case, we have bad disease, we have a good vaccine. The vaccine could be could be used to prevent a disease," he said.
Correction: The 71-year-old man who received the RSV and flu vaccines developed symptoms seven days after receiving the shots. He died on day 22. A previous version misstated the time to symptom onset. Dr. Ann Falsey at the University of Rochester said Guillain-Barre syndrome increases with age with some studies putting the rate at 8 to 12 per 100,000. A previous version misstated the rate.

 Aliver
Aliver