FDA authorizes Covid omicron booster shots for kids as young as 5 years old
The Centers for Disease Control and Prevention still has to issue its recommendations before pharmacies can administer the new shots to kids.
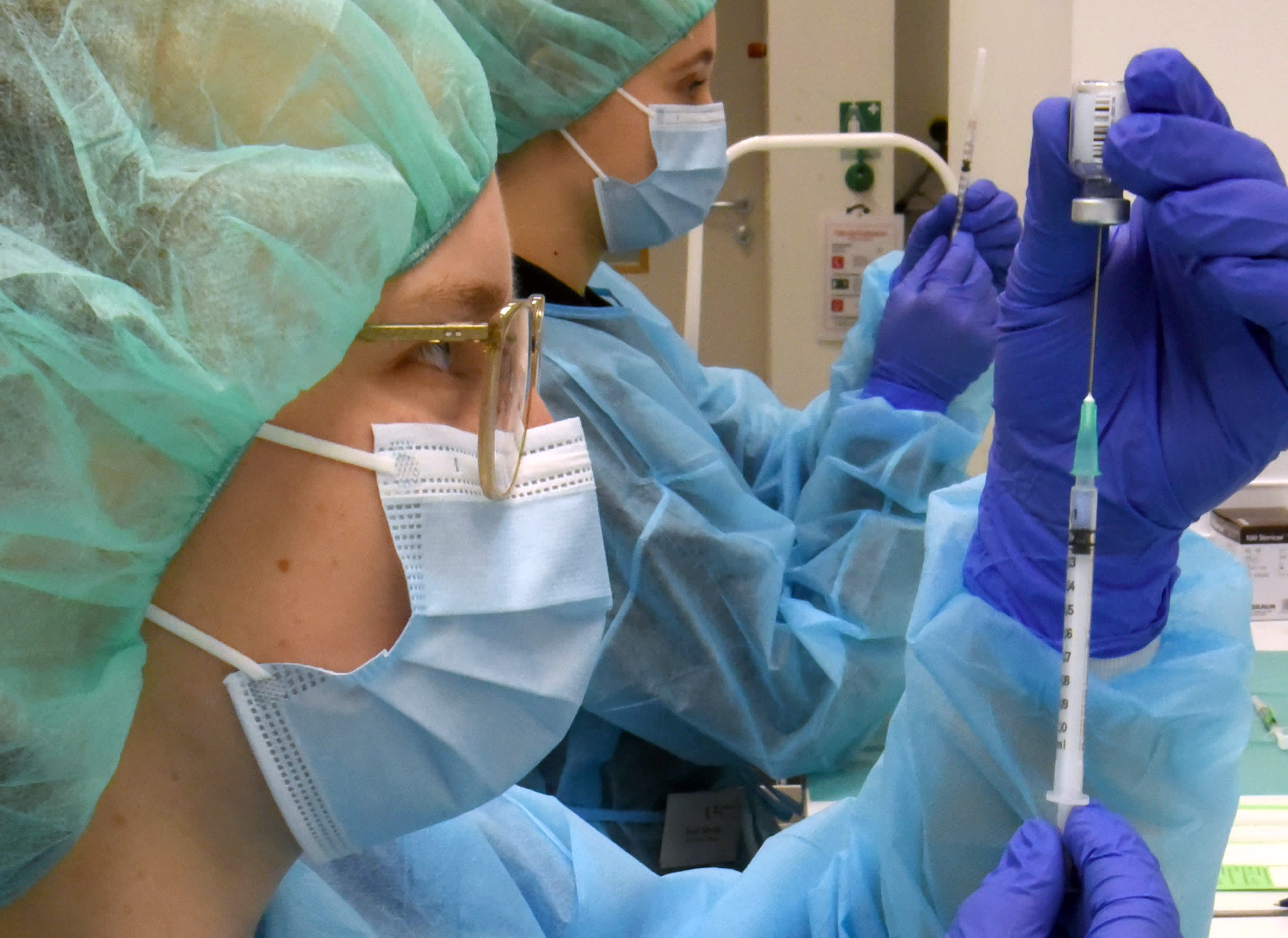
Tatiana Perez, 11, receives a dose of the Pfizer-BioNTech coronavirus disease (COVID-19) vaccine at a vaccination center in San Jose, Costa Rica January 11, 2022.
Mayela Lopez | Reuters
The Food and Drug Administration on Wednesday authorized Covid booster shots that target the omicron variant for preschoolers through elementary school students.
Pfizer's new omicron boosters are now authorized for children ages 5 to 11 and Moderna's shots for kids ages 6 through 17. The shots are administered two months following completion of the two-dose primary series or the most recent booster shot with the first generation vaccines.
The Centers for Disease Control and Prevention still has to issue its recommendations before pharmacies can administer the new shots to kids. The CDC's advisory committee has meetings set for next week when independent vaccine experts will review the available data.
Pfizer's new boosters were cleared for people ages 12 and up in September, while Moderna's were authorized for adults ages 18 and older.
Pfizer, in a statement, said it will ship to up 6 million booster doses for kids within the next week. Vaccinations are expected to start as the school year gets into full swing and just ahead of the holidays when health officials are expecting a spike in infections.
Dr. Peter Marks, head of the FDA's vaccine division, said children face an increased risk of exposure to the virus as they head back to school in person and families return to their pre-pandemic lives.
Although Covid is generally less severe in children than adults, kids do get hospitalized with the disease, Marks said. Health officials are also concerned about the potential risk of long Covid even in children who have had mild illness from the virus, he said.
"We encourage parents to consider primary vaccination for children and follow-up with an updated booster dose when eligible," Marks said.
The FDA hopes the new boosters, which target the dominant omicron BA.5 subvariant, will provide substantially better protection against infection and disease compared to the first generation of Covid shots.
The FDA authorized the BA.5 shots for kids without direct human data on their effectiveness. The agency cleared the boosters based on adult data from a similar shot that targets the omicron BA.1 subvariant. The agency also looked at clinical studies in kids who received the original vaccines as boosters.
The new boosters target omicron BA.5 as well as the original strain of Covid that first emerged in Wuhan, China, in 2019. The FDA hopes the shots will provide durable protection even as the virus continues to evolve because they cover a broad range of mutations.
The first generation of Covid shots were developed in 2020 to target the original strain of Covid. They are no longer providing meaningful protection against infection and mild illness because they do not match the dominant omicron variant, which has mutated to evade the antibodies that block the virus from invading human cells.
More than 11 million Americans ages 12 and older have received the new booster shots so far, according to CDC data.
It's unclear how strong demand for the new shots will be among parents. Just under 50% of people ages 5 and older received a booster shot with the first generation of vaccines.

 Lynk
Lynk