New Covid boosters aren’t better than old shots at neutralizing omicron BA.5, early studies find
Two independent studies suggest the new Covid booster shots don't protect better against omicron BA.5 than the original vaccines.

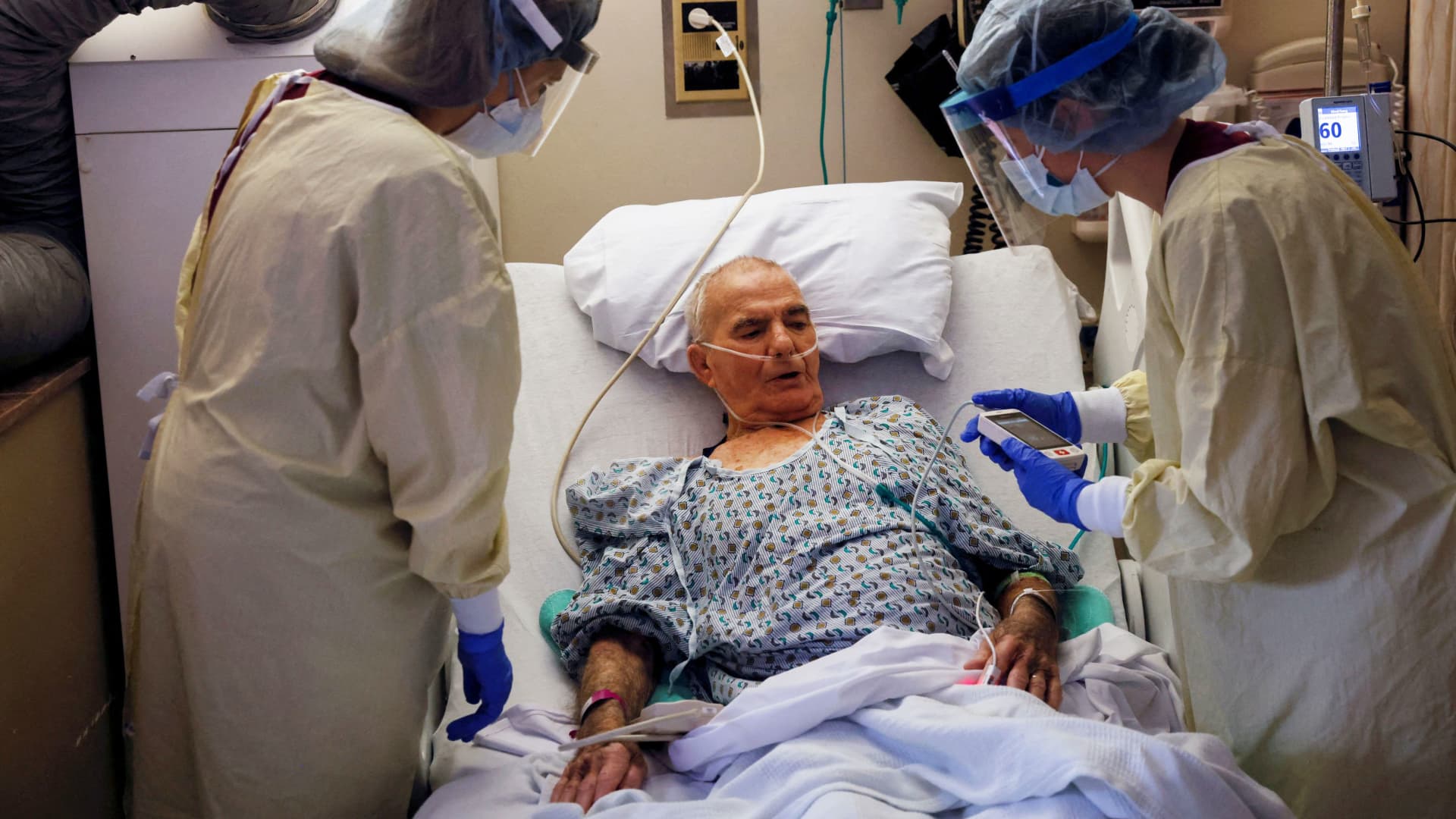
The new COVID-19 booster which includes protection for Omicron at AltaMed Health Services in South Gate on Thursday, October 6, 2022.
Sarah Reingewirtz | MediaNews Group | Los Angeles Daily News via Getty Images
Two studies are raising doubts about whether the new omicron BA.5 booster really will offer better protection against Covid than the first generation shot.
Scientists at Columbia University in New York City found the new boosters did not produce a better antibody response in humans against BA.5 than the first-generation vaccines. A separate study by scientists at Harvard essentially came to the same conclusion.
"It's important to note that the two studies were done independently. They're small studies but there are two of them —it's not just a fluke," said Dr. Dan Barouch, the lead author of the Harvard study. Barouch's lab played a pivotal role in the development of the Johnson & Johnson Covid vaccine.
Both studies were published as preprints, which means neither has undergone peer review by others in the field. They analyzed samples from small groups, 21 people in the Columbia study and 18 in the Harvard study, who received the new boosters and compared them with individuals who received the old vaccine as their fourth shot.
In a statement, the Food and Drug Administration said the preliminary studies are subject to limitations because of their size and data from larger, well-controlled studies are expected soon. Pfizer and Moderna are currently running clinical trials on the new boosters that are expected to read out data later this year.
The FDA said the Harvard and Columbia studies also indicate that the new boosters are generally at least as good as the original vaccines at generating an immune response to omicron BA.5. If the boosters are only modestly better than old shots, it would have positive consequences for public health, the agency said.
"Thus, FDA continues to encourage eligible individuals to consider receiving an updated vaccine to help protect against the currently circulating COVID-19 variants and the wave of COVID-19 that appears to be coming," the agency said.
Dr. Paul Offit, a member of the FDA's independent vaccine advisory committee, said public health officials should be cautious about overselling the shots as a major upgrade.
"We have to be careful when we get in front of the American public and try and sell this vaccine as something that's significantly better when all the evidence we have so far doesn't support that," said Offit, an infectious disease expert at Children's Hospital of Philadelphia, who worked on the team that developed the rotavirus vaccine.
Offit said the boosters work, they're probably just not better than the old shots. In other words, vaccine recipients probably get the same level of protection that would come from a fourth dose with the first generation shots, he said.
"The take home lesson is the people who were in high risk groups and benefit from booster doses as we enter this late fall and early winter – those who are immunocompromised, who have high risk medical conditions, who are elderly — they should get this booster dose," said Offit, who is not affiliated with either study.
The Columbia and Harvard studies were well done, and come from from two of the best virology labs in the country, said Dr. Peter Hotez, co-director of vaccine development at Texas Children's Hospital. But he described the results as preliminary.
"We have to be careful not to draw too many conclusions from it," said Hotez, who also co-led a team that developed a patent-free vaccine called Corbevax that India authorized for use last December.
Hotez said there should also be investigations into how the boosters perform against emerging omicron subvariants such as XBB and BQ.1., as the currently dominant BA.5 declines in circulation. It could be the case that the new boosters perform better against these emerging variants than the first generation shots do, Hotez said.
The White House, the FDA and the Centers for Disease Control and Prevention have repeatedly expressed confidence that the new boosters will provide better protection than the old shots. This is because they are bivalent shots that directly target the dominant variant, omicron BA.5, as well as the original Covid strain that emerged in China in 2019.
The first generation vaccines, on the other hand, are monovalent shots that only target original the Covid strain, which scientists call wild type. As the virus has evolved away from the wild type, the monovalent shots are no longer providing meaningful protection against infection and mild illness.
They do still generally prevent hospitalization, though this protection is also declining over time.
"It is reasonable to expect based on what we know about immunology and the science of this virus that these new vaccines will provide better protection against infection, better protection against transmission and ongoing and better protection against serious illness," Dr. Ashish Jha, head of the White House Covid taskforce, told reporters in September.
White House chief medical advisor Anthony Fauci also said at the time that the boosters should provide better protection than the old shots, though he said it was difficult to predict how much more effective they would be. This is because the Food and Drug Administration authorized the bivalent shots in September without direct human immune response or efficacy data on the BA.5 boosters.
Instead, the FDA relied on human data from a similar vaccine that targets the first version of omicron, BA.1. Pfizer and Moderna were originally developing their new boosters against BA.1, but the FDA asked the companies to switch gears and target BA.5 as that subvariant became dominant over the summer.
As consequence, Pfizer and Moderna did not have time to launch clinical trials and present data on the BA.5 boosters before authorization. The FDA also relied on animal studies that looked directly at the immune response induced by the BA.5 shots.
The agency was acting with urgency to get the new boosters out by the fall in the hope that they would help head off a major Covid surge.
The scientists at Columbia and Harvard said their studies suggest that a phenomenon called "immune imprinting" may pose a challenge to new boosters. This means your immune system is already primed by the monovalent shots to recognize wild type Covid, which can make it difficult to train your body to recognize and attack new strains.
Hotez said it might be possible to overcome immune imprinting, if it is in fact a problem, by giving a second dose of the BA.5 shot at some point. In other words, the booster might not push a stubborn immune system trained to recognize wild type to shift gears and attack a new variant the first time around. But a second dose could convince it to produce antibodies against BA.5.
But Offit said the antibodies that protect against mild illness are inherently short lived. The real focus should be on preventing severe disease and hospitalization, which is what the vaccines are successfully doing.
"You're going to get mild illness probably again and again with this virus, as is true for all short incubation period, mucosal respiratory viruses — live with it," Offit said. "We're going to have to learn to live with it because that's the only thing that is achievable — keeping people out of the hospital."

 Koichiko
Koichiko