Pfizer says third Covid vaccine shot for kids under 5 is 80% effective against omicron
A third dose of the vaccine elicited a strong immune response and was well tolerated by the kids, according to Pfizer.
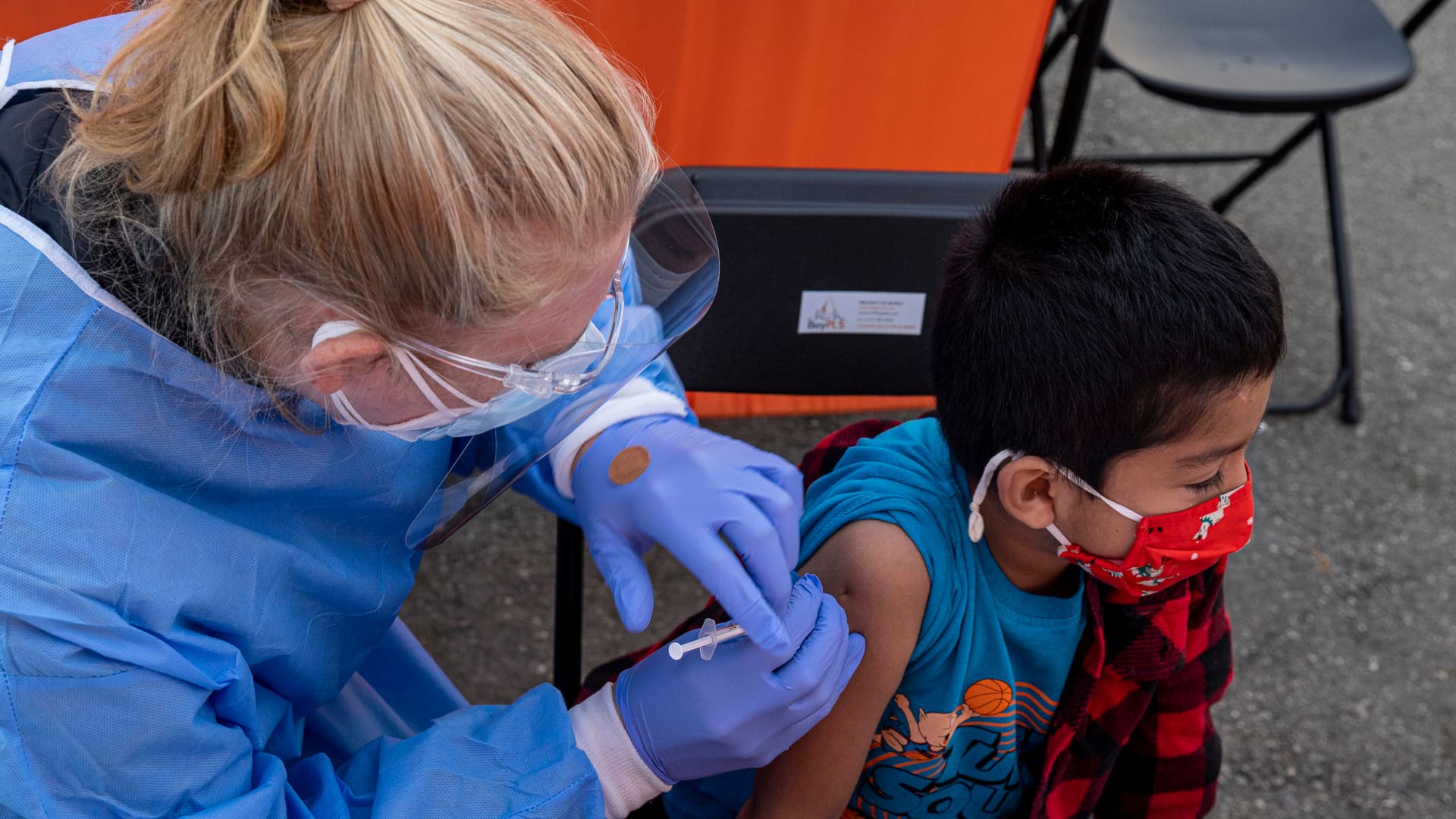
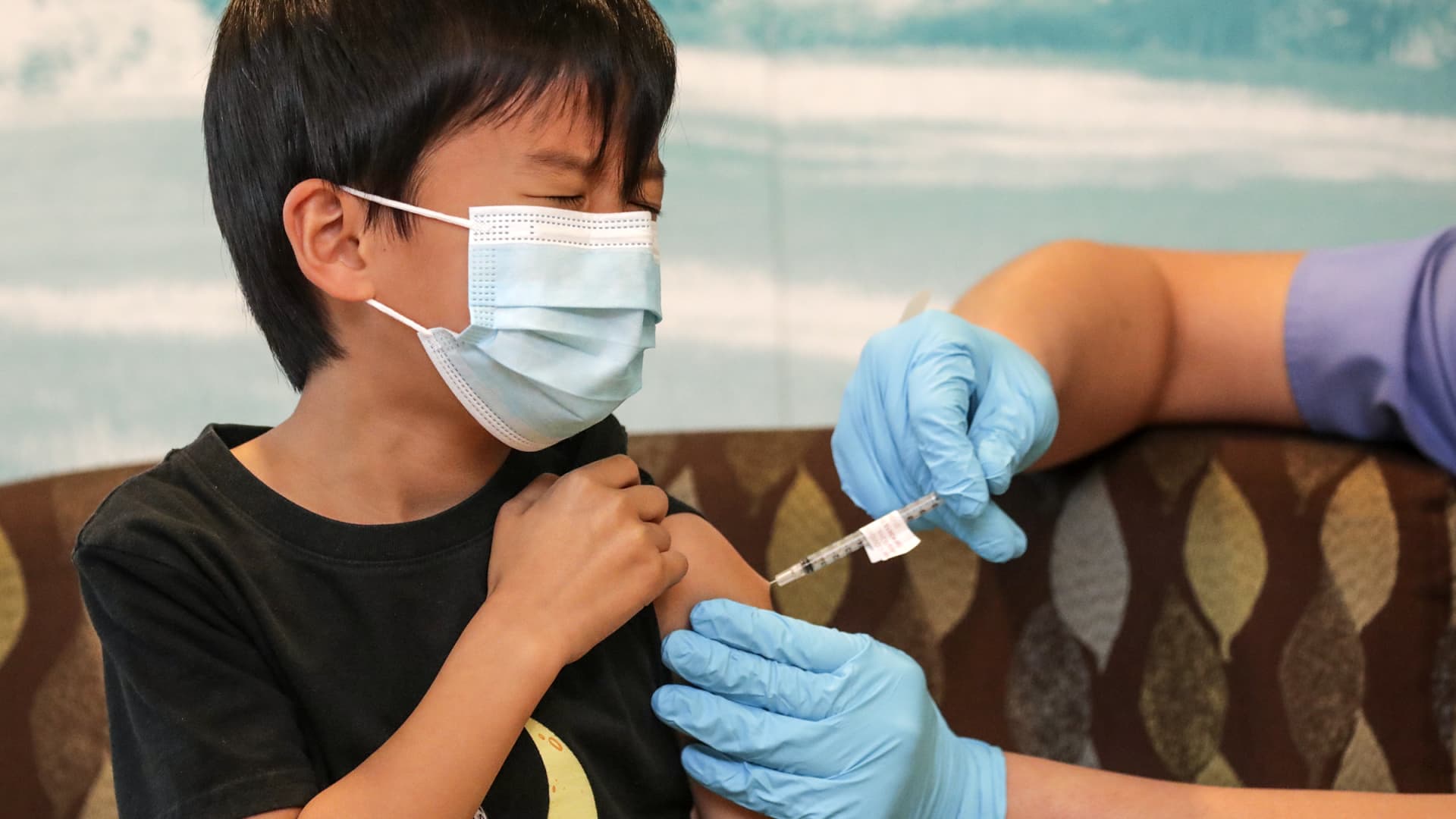
A healthcare worker administers a Pfizer-BioNTech Covid-19 vaccine to a child at vaccination site in San Francisco, California, U.S., on Monday, Jan. 10, 2022.
David Paul Morris | Bloomberg | Getty Images
Pfizer and BioNTech's three-dose Covid vaccine for children 6 months to 5 years old was 80% effective at preventing illness during the omicron wave, according to preliminary clinical trial results released Monday.
A third dose of the vaccine elicited a strong immune response and was well tolerated by the kids with a majority of the side effects mild to moderate, according to the companies.
BioNTech CEO Ugur Sahin said the companies plan this week to complete their application asking the Food and Drug Administration to authorize the vaccine for emergency use. Pfizer CEO Albert Bourla said he hopes the vaccine will be available to younger kids as soon as possible.
Hours after Pfizer released the data, the FDA announced its committee of independent vaccine experts would meet June 15 to discuss Pfizer's and Moderna's applications to have their shots authorized for infants through preschoolers.
The FDA committee will review the data in a meeting open to the public and will then make a recommendation on whether the agency should authorize the shots. Though the FDA isn't obligated to follow the committee's recommendation, it usually does.
The FDA, in a statement, said it expects to complete its review Pfizer's and Moderna's vaccines for infants and preschoolers within days of each other.
"We know parents are anxious for us to determine if these vaccines are safe & effective," the agency said in a post on Twitter. "We are working as quickly as possible to carefully review all the data."
The safety data for the Pfizer vaccine is based on 1,678 children under age 5 who received a third shot at least two months after the second dose when omicron was the main variant in circulation. Pfizer examined a subset of children to determine the immune response of kids under age 5. The 80% effectiveness against omicron is based on a preliminary analysis. Bourla, in a Twitter post Monday, said Pfizer would release the final analysis as soon as it's finished.
Kids under age 5 receive 3-microgram shots, one-tenth the dosage level for adults.
Pfizer and the FDA had originally sought to fast-track authorization of the first two doses in February so parents could start getting their kids vaccinated while clinical trial results from the third shot were still pending.
However, Pfizer delayed its application to wait on data from the third dose after the first two shots were only 30% to 40% effective, Bourla said in a podcast interview last month.
Children under age 5 are the only group in the U.S. that is not yet eligible for vaccination. Parents have been waiting months for the FDA to authorize the shots.
During the massive wave of omicron infection during the winter, children younger than age 5 were hospitalized with Covid at five times the rate of the pandemic peak, according to the Centers for Disease Control and Prevention. About 75% of kids under age 11 had been infected with Covid as of February, according to CDC data.
Moderna has also asked the FDA for emergency use authorization of its two-dose vaccine for children under age 6. Its vaccine was about 51% effective against infection from omicron in children under age 2, and about 37% effective for kids ages 2 to under 6 years old. However, Moderna said the antibody levels induced by the vaccine should translate into high levels of protection against severe illness.

 Aliver
Aliver