U.S. scientists are enrolling nearly 40,000 patients in 4-year $1.2 billion study of long Covid
The study, Recover, aims to complete enrollment of 40,000 people and launch clinical trials on potential treatments by the end of the year.

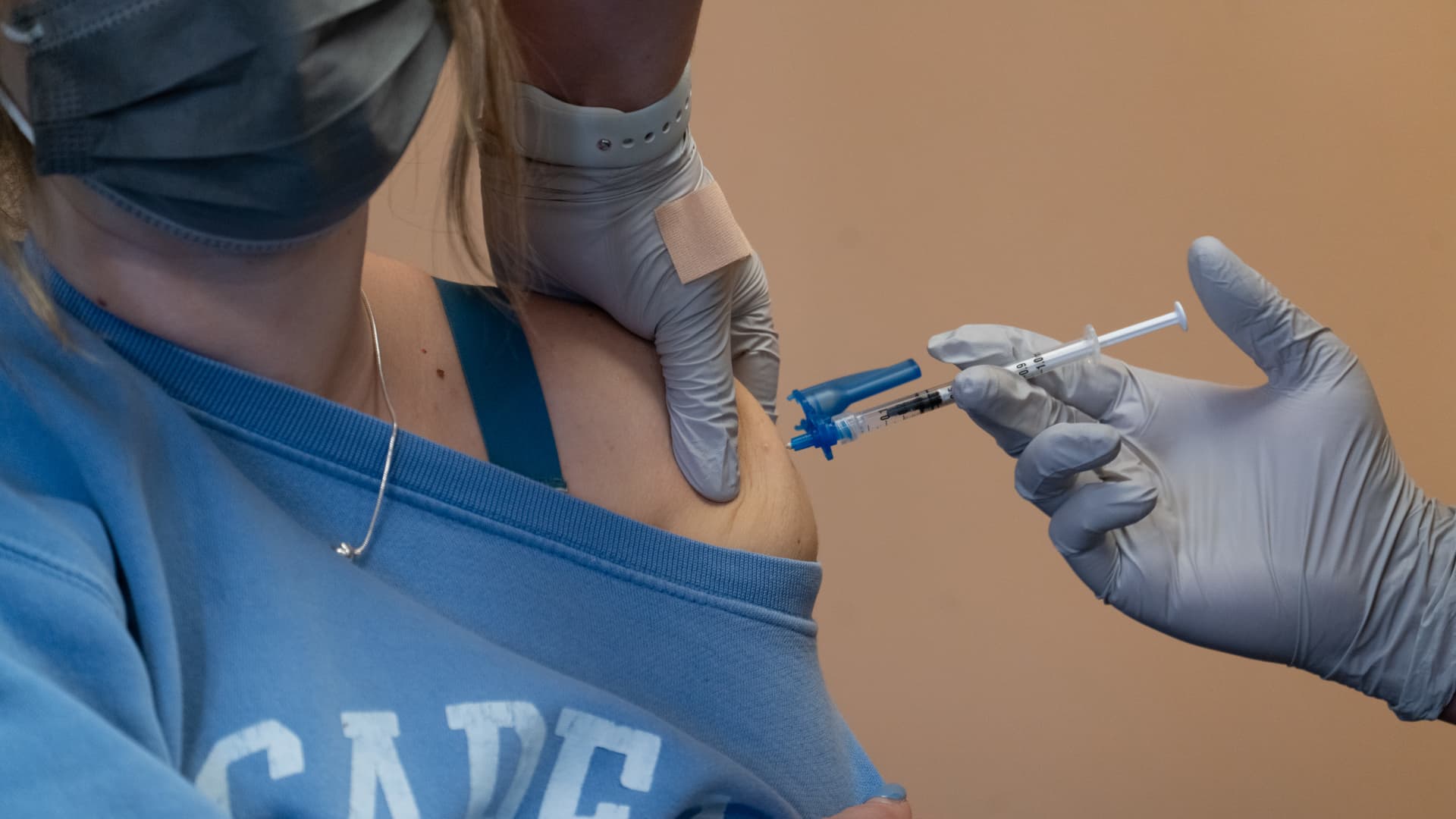
A healthcare worker administers a Covid-19 test at testing site in San Francisco, California, U.S., on Monday, Jan. 10, 2022.
David Paul Morris | Bloomberg | Getty Images
The National Institutes of Health is rolling out one of the largest studies in the world to understand long Covid in a high-stakes effort to find definitive answers about a multitude of seemingly unrelated and sometimes debilitating symptoms that have plagued patients and confounded physicians.
The $1.15 billion taxpayer-funded study, called Recover, aims to enroll nearly 40,000 people by the end of this year. It will follow those participants over four years, comparing people with Covid to those who've never had it, with the goal of identifying all the long-term symptoms and finding out how the virus is causing them. The Patient-Led Research Collaborative said there were more than 200 long Covid symptoms across 10 organ systems, according to a study published last year in The Lancet.
It's a massive undertaking, and expectations are high. The size of the budget, breadth, depth and scope of the study are rarely seen in scientific studies.
The study's conclusions could play a pivotal role in developing diagnostic tests and finding treatments for patients who remain sick months after contracting Covid-19. If the scientists can produce clinical definitions of the various long-term illnesses associated with the virus, patients will stand on firmer ground when trying to convince health insurers to cover their treatments and getting disability claims approved.
Dr. Walter Koroshetz, who serves on Recover's executive committee, said the study has been designed to investigate long Covid from every possible angle and provide definitive answers. But Koroshetz acknowledged that even a study this size will face major challenges in delivering on such ambitious goals.
"I'm worried that this is not an easy answer. The post-infectious persistent symptoms that go on to chronic fatigue syndrome have defied anybody's explanation," said Koroshetz, the director of the National Institute of Neurological Disorders and Stroke.
Enrollment and clinical trials
The Recover study aims to complete enrollment of more than 17,000 adults by September and 20,000 children by the end of the year, according to Dr. Stuart Katz, who is coordinating the nationwide rollout of the Recover study at its central hub at New York University Langone Health. The study will have research teams at more than 30 universities and medical institutions across the U.S.
As of this week, 5,317 adults and 269 children have been enrolled, taken together about 15% of the total population of nearly 40,000, according to Katz, a cardiologist who studies congestive heart failure. Katz caught Covid in December 2020 and suffered symptoms for about a year.
The National Institutes of Health is also planning to launch a "suite of clinical trials" on possible treatments in the coming months, according to Dr. Gary Gibbons, director of the National, Heart Lung and Blood Institute. Gibbons said NIH is in active discussions with the pharmaceutical industry on studying whether antivirals and other interventions can prevent or treat long Covid.
"These are exploratory with companies that have agents that may go before the FDA for approval," Gibbons said. "There's an interest both for public-private collaboration in this space and and we're very hopeful that something will emerge in the next several months."
However, Gibbons said NIH will likely need more funding from Congress for the trials given scope and complexity of the problem.
"We would anticipate to really fully do the clinical trial portfolio that patients with long Covid deserve, it probably will exceed $1.15 billion initial allocation that Congress awarded," Gibbons said.
Unanswered questions
While the public uses long Covid for shorthand, the scientific name is post-acute sequelae of Covid, or PASC. Researchers believe it is not a single disease but several distinct illnesses affecting many organ systems.
Scientists still do not know how the virus triggers such a wide spectrum of symptoms that can persist months after the initial infection, why some of these symptoms show up in some patients but not in others, or what exactly the risk factors are for developing them.
"Everyone's immune system is different, so everyone's going to respond to a novel virus in a different way," said David Putrino, a physiotherapist and director of rehabilitation innovation at Mount Sinai Health System in New York City. Putrino has helped treat long Covid patients since the early days of the pandemic in 2020. Mount Sinai's Icahn School of Medicine is one the institutions participating in Recover.
Putrino said many patients come to Mount Sinai for treatment suffer cognitive impairments that are similar to traumatic brain injuries, commonly referred to as brain fog, in which they struggle with speech fluency and making plans to deal with life's daily challenges. They can also often have abnormal heartbeat, tingling sensations, painful cramps and feelings of anxiety.
Any form of physical or mental exertion worsen these symptoms. As a consequence, about 60% of the long Covid patients at Mount Sinai struggle to continue at their jobs, Putrino said. They either had to shift to part-time work from full time, retire early or became unemployed. Almost all of the patients report a deterioration in their qualify of life due to their symptoms, he added.
The nation's health agencies do not yet know exactly how many people suffer from the condition. The answer to that question, which Recover hopes to shed more light on, could have major implications for the nation's health and economy.
The Centers for Disease Control and Prevention, in a study that examined nearly 2 million patient records, found that one in five Covid survivors ages 18 to 64 and one in four ages 65 and older developed a health problem that could be related to long Covid. If the findings prove accurate for the broader population, millions of people in the U.S. may have some form of the condition.
People who survived the virus were twice as likely to develop respiratory conditions or a pulmonary embolism, according to the CDC study. The authors said long Covid can impair a person's ability to work which could have economic consequences for their families.
The severity and duration of patients' long Covid symptoms vary widely, Katz said. The population of people permanently disabled by long Covid is likely a fraction of those who have some form of the condition, he said. Still, there's likely a very large number of people who have a disability from long Covid given the reality that at least 87 million people in the U.S. have contracted the virus at some point, Katz said.
How Recover will work
With so many unanswered questions, physicians don't have a precise way to diagnose patients with long Covid. Treatments at this point are mostly managing symptoms, not addressing the underlying cause of the illnesses, Putrino said. Scientists need to define the different types of long Covid so they can tailor treatments to individual patients, he added.
The challenge with diagnosing and treating patients with long Covid is that many of the symptoms are also associated with other diseases, said Katz. Recover contains control groups, people who have never had Covid, so scientists can define which symptoms are actually occurring more often in people who do have a history of infection, Katz said.
All the participants in Recover will undergo a battery of lab tests, vital signs and physical assessments, as well as a survey of symptoms and underlying health conditions among many other questions at enrollment and at regular intervals throughout the study. Smaller populations of participants will undergo more intense evaluations that include electrocardiograms, brain MRIs, CT scans and pulmonary function tests.
The scientists aim to identify clusters of symptoms associated with various abnormalities in the lab tests and uncover the mechanisms in the body causing those symptoms through advanced imaging, Katz said. Abnormalities found in lab tests, blood samples for example, that are associated with long Covid could serve as the basis for future diagnostic tests, he said.
By defining the different types of long Covid, the study will also guide clinical trials by providing a clearer idea of what treatments might prove most effective at targeting the underlying causes.
"Clinicians really need us to clarify what is the clinical spectrum, the definition of long Covid — that's critical to treating it," Gibbons said. "If you're going to do a clinical trial, you really want to know that you might treat brain fog different from the cardiopulmonary symptoms," he said.
Recover will also analyze tens of millions of electronic patient health records and study tissue samples from autopsies of people who had Covid when they died. All of the Recover data will go into a database that investigators at sites across the country can use in research on specific aspects of long Covid that they can pitch to Recover's leadership.
Dr. Grace McComsey, the principal investigator for the Recover site at Case Western Reserve University in Cleveland, said the study design will allow her team to access a large pool of patient data that they otherwise wouldn't have the time or resources to collect on their own. McComsey, an infectious disease expert who researched HIV before the pandemic, has submitted a concept with her team to look at how the virus is causing inflammation in patients.
"You'll be able to access a lot of data, lots of samples on patients that otherwise I can't do from my own site. It will take me obviously a lot of time and a lot of resources that I don't have," McComsey said. "The huge amount of data and a huge amount of patients. I think it's definitely a big plus in Recover."
Criticism of time frame
However, the pace of the federal government's efforts to address the long-term health impact of Covid has come under criticism. Some of the nation's leading health experts described research into long Covid as "achingly slow," according to a March report whose authors included several former members of President Joe Biden's Covid transition team, including Zeke Emanuel.
It's been more than a year and a half since Congress OK'd $1.15 billion to study the long-term impact of Covid in December 2020. Francis Collins, NIH director at the time, announced in February 2021 the launch of a nationwide study. The following May, NIH awarded $470 million to New York University Langone to set up the observational part of the study led by Katz and his team.
Koroshetz acknowledged the frustration with the pace of the research, but he said the study is designed through its size and scope to answer questions smaller studies cannot.
"We put this together to not miss anything," Koroshetz said. "It's kind of like a battleship. That's part of the problem."
Although Recover will follow participants for four years, researchers will publish their findings throughout the duration of study, Katz said. The first report, based on the initial assessment of participants, should publish shortly after enrollment is complete, he said.
"In comparison with other large multisite studies, this was all done at breakneck speed because there was a recognition that there is an urgent public health need," said Katz.
Putrino said NIH-funded research is usually slow, risk averse and normally doesn't lead to rapid implementation of treatments that help patients. He said NIH typically doesn't invest in high-risk research because it doesn't want to be perceived as gambling with taxpayer money. Putrino said his team applied for a Recover grant in December 2021 and haven't heard back yet.
He said NIH should act more like industry by moving quickly to invest in high-risk research that can lead to disruptive innovations.
"The NIH has the capacity to follow a process similar to industry — it's not typical but they can do it," said Putrino, who was one of the authors on the March report that criticized the pace of the federal government's long Covid efforts. "We need a high-risk investment right now," he said.
In April, President Biden directed Health and Human Services Secretary Xavier Becerra to develop a national research action plan on long Covid in collaboration with the secretaries of Defense, Labor, Energy and Veterans Affairs. HHS is supposed to have the plan ready next month, according to Biden's directive.
JD Davids, a patient advocate, said the NIH should model the federal response on long Covid after its success in researching and developing HIV treatments. That includes creating a central office at NIH with budgetary authority, similar to the Office of Aids Research, that develops a strategy every year with input from patients on how to use funds for research, said Davids, a member of the Patient-Led Research Collaborative.
Koroshetz and Gibbons said Recover is moving as quickly as possible to get clinical trials on treatments started. "We're not going to wait four years and then do the trials. We're going to whatever rises to the top in terms of ideas," Koroshetz said.
Gibbons said NIH can't provide a timeline right now on how long the clinical trials will take. Although NIH is soliciting concepts, it doesn't have any finished plans for how the trials will proceed yet, said.
"It's probably not a satisfying answer, but we can only move at the pace of the science," Gibbons said. "If you establish the protocol, you have to enroll participants and you have to let the protocol play out. We don't have a protocol yet in hand."

 JaneWalter
JaneWalter