CDC head Dr. Walensky on fast-tracking new omicron-specific boosters: The consequences could be worse 'if we wait'
CDC director Dr. Rochelle Walensky says new BA.5-specific Covid booster shots need to roll out quickly, even if they aren't fully tested in humans yet. Here's why.

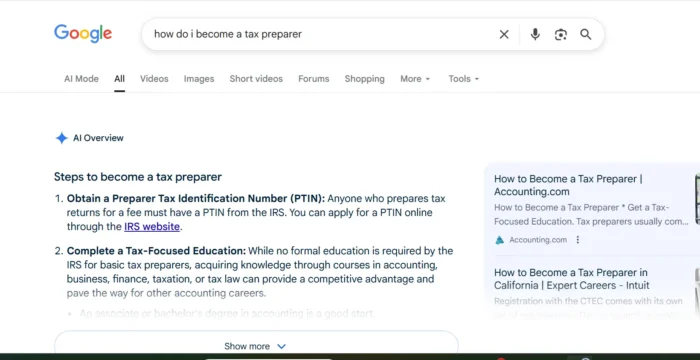
The omicron-specific booster shots set to arrive within the next week are being fast-tracked, and just got approved by the U.S. Food and Drug Administration before being fully tested in humans.
There's a good reason why, according to Centers for Disease Control and Prevention director Dr. Rochelle Walensky.
"If we wait for those data to emerge in human data, not just mice data, we will be using what I would consider to be a potentially outdated vaccine," Walenksy said on the "Conversations on Health Care" radio show last week. That could have severe consequences for the nation ahead of a projected Covid surge in the fall and winter, she added.
The FDA authorized new boosters from Pfizer and Moderna on Wednesday, for most Americans who have completed their primary vaccination series. The agency approved Pfizer's updated booster for people ages 12 or older, and Moderna's updated shot for people ages 18 and older. Shots will be available once the CDC provides guidelines for their use, which is expected in the coming days.
Both shots have yet to complete human trials. The FDA based its decision off real-world evidence of existing Covid vaccine safety, plus clinical trial data of earlier bivalent vaccines targeting older forms of omicron and current lab data on the BA.5 shot in mice.
The approach is nothing new: The agency already uses a similar strategy with flu shots, which are safely updated each year without human testing to keep up with mutating flu viruses.
"The FDA has extensive experience with reviewing strain changes in vaccines, as is done with the annual flu vaccine," FDA commissioner Robert Califf wrote on Twitter last week.
The agency's approval strategy has stirred debate among scientists, with some suggesting that people wait for human test results before getting one of the boosters themselves.
"For the FDA to rely on mouse data is just bizarre, in my opinion," John Moore, an immunologist at Weill Cornell Medicine in New York, told NPR earlier this month. "Mouse data are not going to be predictive in any way of what you would see in humans."
But the country urgently needs the updated shots' targeted layer of protection against BA.5, which currently accounts for nearly 88% of confirmed U.S. cases, Walensky said: The fast-tracked approval could help people get those doses before the virus potentially gives way to new variants, rendering the BA.5-specific shots "outdated."
"I believe it is best to use a vaccine that's tailored for the variant that we have right now," Walensky said, noting that the updates to the boosters' components are "very, very small," and aren't expected to affect the safety of the shots.
Califf emphasized the same point on Twitter last week, writing: "Real world evidence from the current mRNA COVID-19 vaccines, which have been administered to millions of individuals, show us that the vaccines are safe. As we know from prior experience, strain changes can be made without affecting safety."
Approving the new boosters as soon as possible should be a priority heading into the fall and winter, when BA.5 could wreak more havoc on the U.S., Walensky emphasized: "I really would love to be ahead of the variant this season."
Update: This story has been updated to reflect the FDA's approval of bivalent Covid booster shots from Pfizer and Moderna.
Sign up now: Get smarter about your money and career with our weekly newsletter
Don't miss:
Omicron-specific Covid vaccines could finally be here this fall—here's what you need to know

 UsenB
UsenB