FDA advisory committee to discuss future of Covid boosters
An FDA advisory committee will discuss Covid-19 boosters for the coming months and what factors would make it necessary to update the shots to target specific variants.
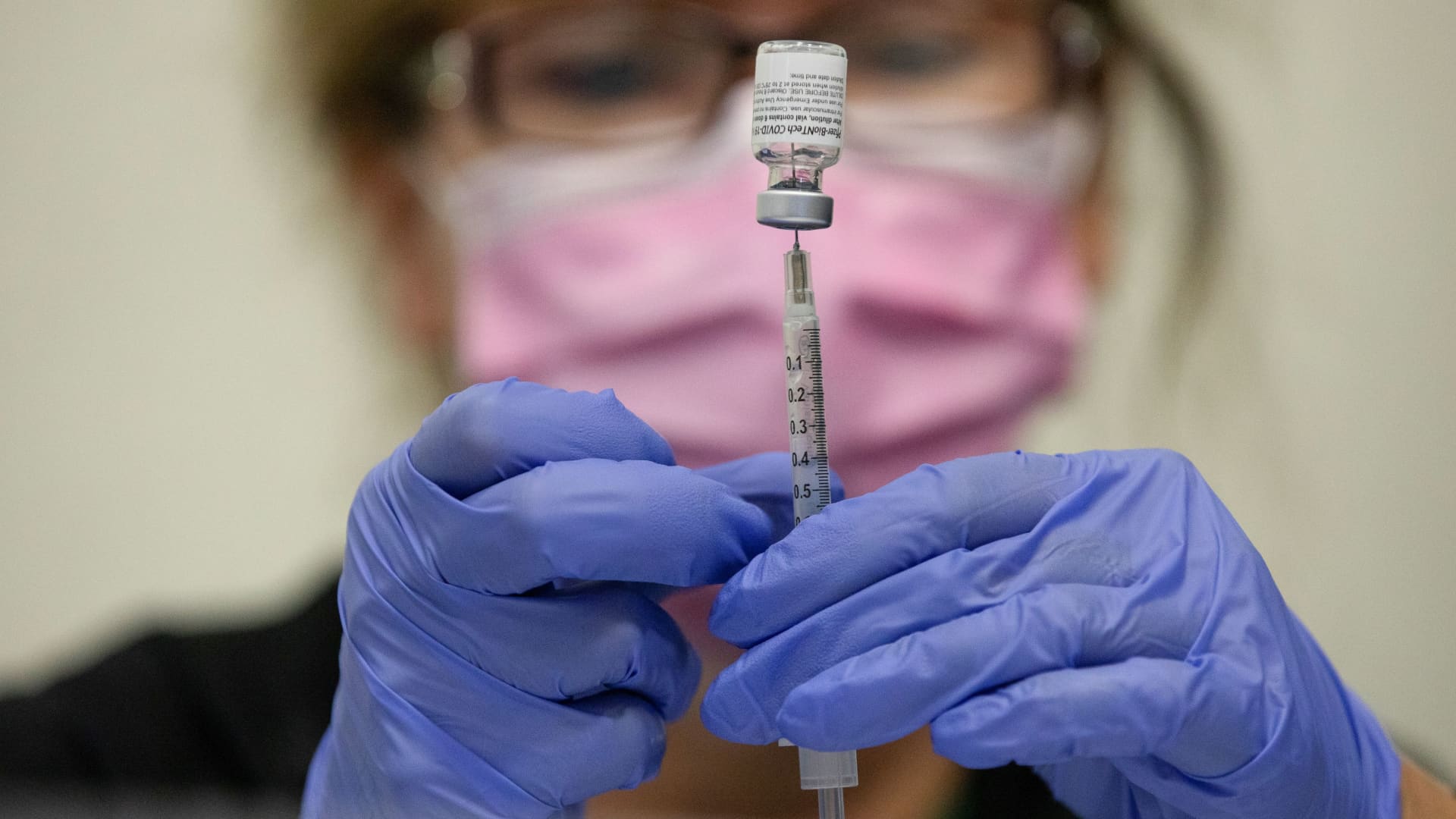
A nurses fills up syringes for patients as they receive their coronavirus disease (COVID-19) booster vaccination during a Pfizer-BioNTech vaccination clinic in Southfield, Michigan, September 29, 2021.
Emily Elconin | Reuters
A Food and Drug Administration advisory committee will meet next month to discuss the future of Covid-19 booster shots in the U.S. and whether the vaccines should be updated to target specific variants.
The FDA's Vaccines and Related Biological Products Advisory Committee will meet April 6 to debate the timing of Covid boosters for the coming months as well as when the shots should be updated to target specific variants. They haven't scheduled a specific vote nor are they expected to discuss Pfizer or Moderna's recent applications for fourth Covid vaccine doses.
Public health experts and the vaccine makers have said Covid will eventually become a seasonal virus like the flu, which has higher transmission during the winter months and then recedes when the weather turns warm again. The CEOs of Pfizer and Moderna have both said annual vaccinations against Covid will be necessary similar to the flu, particularly for the elderly and those with underlying conditions.
Ever year, the FDA advisory committee decides which flu vaccine should be administered in the U.S. based on what strain is circulating and other factors. The committee will likely take a similar approach to Covid vaccines moving forward.
"Now is the time to discuss the need for future boosters as we aim to move forward safely, with COVID-19 becoming a virus like others such as influenza that we prepare for, protect against, and treat," said Dr. Peter Marks, the head of the FDA's vaccine safety group.

 KickT
KickT