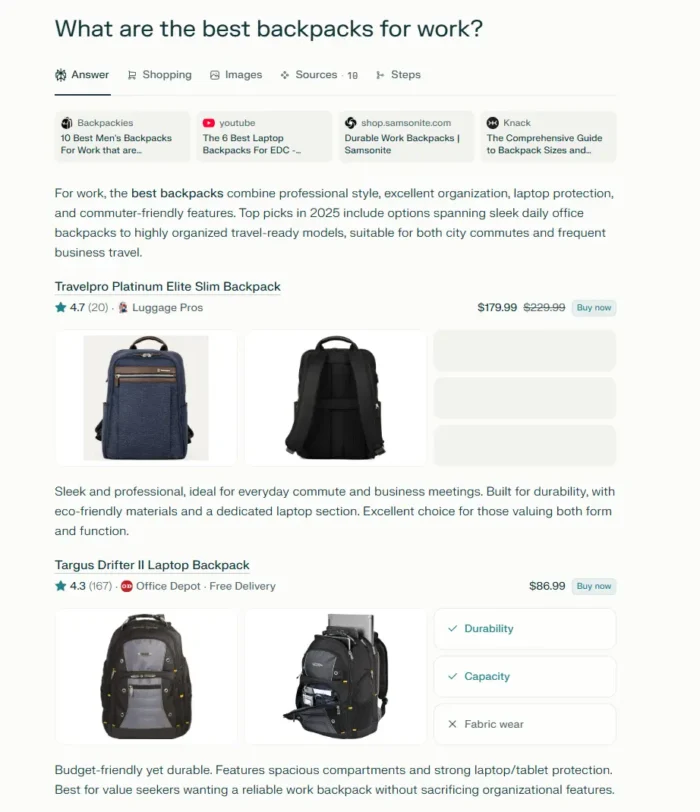
FDA approves over-the-counter sales of lifesaving opioid overdose treatment Narcan nasal spray
The FDA's decision means people will be able to buy the 4mg nasal spray without a prescription in supermarkets, convenience stores and vending machines.

Fentanyl forum attendees could visit a table where Narcan kits were being offered free at Northwood High School in Silver Spring, Maryland on February 25, 2023.
Michael S. Williamson | The Washington Post | Getty Images
The Food and Drug Administration on Wednesday approved sales without a prescription of the nasal spray Narcan to reverse opioid overdoses, a decision that promises to significantly expand access to the lifesaving treatment.
The FDA's decision means people will be able to buy the 4 milligram nasal spray in supermarkets, convenience stores, gas stations, vending machines and online. Emergent BioSolutions, the manufacturer, has said Narcan should be available without a prescription by late summer.
FDA Commissioner Dr. Robert Califf, in a statement, said the agency is encouraging the company to make the nasal spray available as soon as possible at an affordable price.
Narcan reverses fatal overdoses by blocking the effect that opioids have on the nervous system. The nasal spray must be administered as soon as an overdose is suspected.
Two nasal spray devices typically come in a single package. The first dose should be administered in one nostril of the person suffering an overdose and then 911 should be called, according to the instructions. If the person is still unresponsive after two to three minutes, the second dose should be administered.
After the FDA's decision, Walgreens said it will offer over-the-counter Narcan later this year in-store and online nationwide.
"Delivering access to this lifesaving medication that can reverse the effects of an overdose if administered in time is imperative and Walgreens is already working with suppliers to bring this OTC medication to shelves," said Zoe Krey, a Walgreens spokesperson.
Join CNBC's Healthy Returns on March 29th, where we'll convene a virtual gathering of CEOs, scientists, investors and innovators in the health care space to reflect on the progress made today to reinvent the future of medicine. Plus, we'll have an exclusive rundown of the best investment opportunities in biopharma, health-tech and managed care. Learn more and register today: http://bit.ly/3DUNbRo
The FDA said in November it was considering approving naloxone products, the generic name for medications that reverse opioid overdoses, for use without a prescription. The push to make naloxone easier to access is part of the agency's efforts to fight the opioid crisis.
The Trump administration first declared the opioid epidemic a public health emergency in 2017. The Biden administration has renewed the public health emergency every 90 days.
More than 564,000 people died from opioid overdoses between 1999 and 2020, according to the Centers for Disease Control and Prevention. The first wave of the epidemic began in the 1990s with prescription opioids, followed by an increase in deaths from heroin starting in 2010.
Deaths from synthetic opioids such as fentanyl have increased significantly since 2013. More than 71,000 people died from synthetic opioids in 2021, a 18% increase over the year prior, according to CDC estimates.

 Tfoso
Tfoso