FDA withdraws Covid antibody treatment Evusheld because it's not effective against 93% of subvariants
Many take Evusheld as an additional layer of protection because the vaccines do not trigger a strong immune response for them.
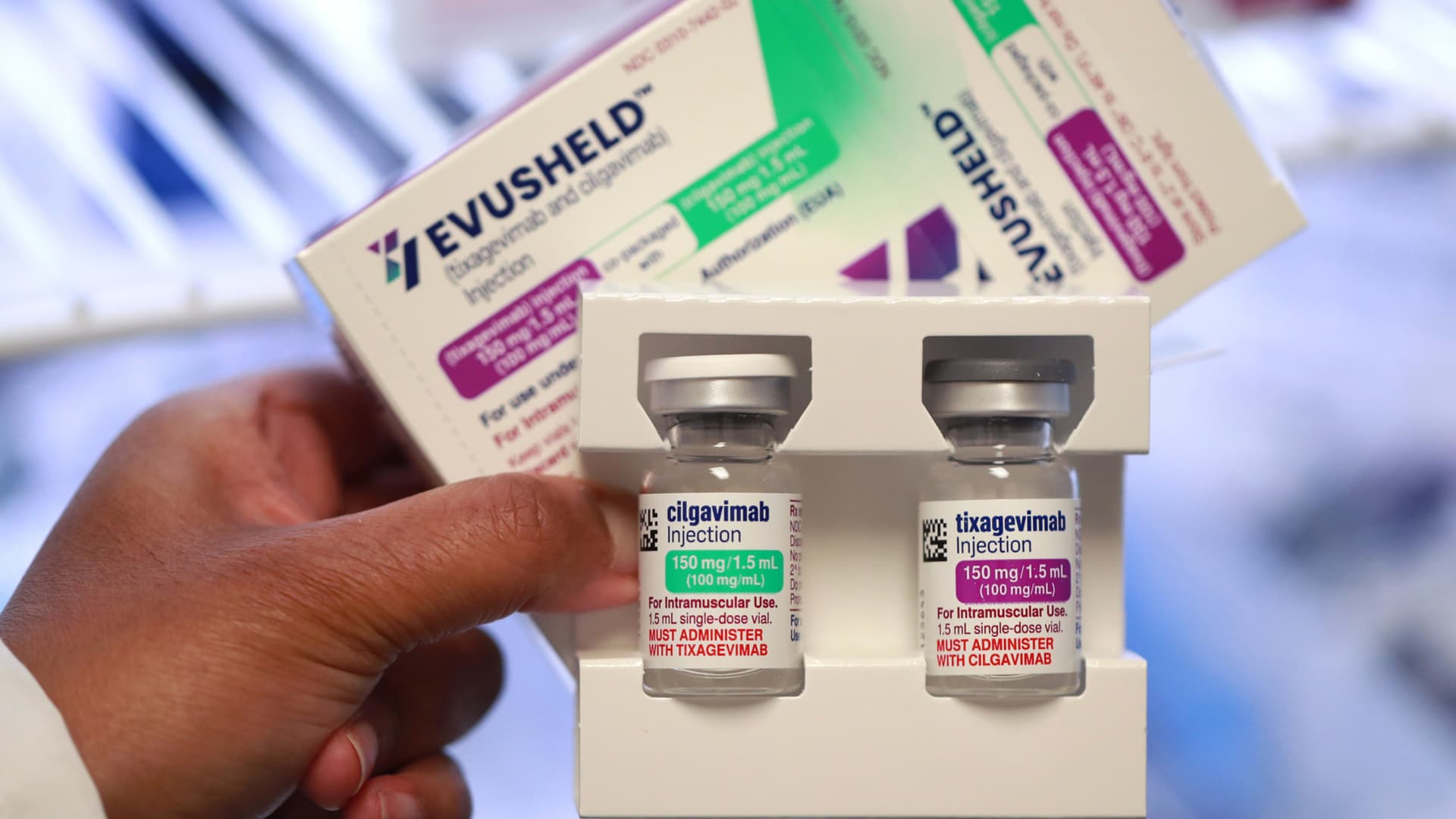
Evusheld (tixagevimab and cilgavimab) injection, a new COVID-19 treatment that people can take before becoming symptomatic. (Chris Sweda/Chicago Tribune/Tribune News Service via Getty Images)
Chris Sweda | Tribune News Service | Getty Images
The Food and Drug Administration on Thursday pulled its authorization for AstraZeneca's Evusheld, an antibody injection that people with weak immune systems relied on for additional protection against Covid-19.
The FDA pulled Evusheld from the market because it is not effective against more than 90% of the Covid subvariants that are currently circulating in the U.S.
The omicron XBB.1.5 subvariant, which is adept at evading antibodies that block infection, has quickly risen in the U.S. and is now causing 49% of new cases, according to data from the Centers for Disease Control and Prevention.
Evusheld is also not effective against the BQ.1, BQ.1.1 and XBB subvariants. Taken together with XBB.1.5, versions of Covid that are resistant to Evusheld now represent nearly 93% of new cases in the U.S.
"Today's action to limit the use of Evusheld prevents exposing patients to possible side effects of Evusheld such as allergic reactions, which can be potentially serious, at a time when fewer than 10% of circulating variants in the U.S. causing infection are susceptible to the product," the FDA said in a statement Thursday.
People with compromised immune systems, such as cancer chemotherapy and organ-transplant patients, are some of the groups most vulnerable to severe disease from Covid. Many take Evusheld as an additional layer of protection because the vaccines do not trigger a strong immune response for them.
The decision to pull Evusheld comes more than a month after the FDA withdrew an antibody treatment called bebtelovimab because it was not effective against the BQ.1 and BQ.1.1 subvariants.
Evusheld is taken as a preventive measure before exposure to Covid. It is a combination of antibodies, cilgavimab and tixagevimab, taken as two injections every six months.
Just over one million doses of Evusheld have been distributed in the U.S. since the FDA authorized the injections in December 2021, according to data from the Health and Human Services Department. About 720,000 of those doses have actually been administered to patients.
More than 7 million adults in the U.S. have a compromised immune system. They represented about 12% of Covid hospitalizations, despite making up just 3% of the population, according to a study from the CDC that looked at data from 10 states.
There is currently no replacement for Evusheld. Dr. Ashish Jha, head of the White House Covid task force, has blamed Congress for the dwindling number of treatments. He said lawmakers' failure to pass additional Covid funding means there isn't money to invest in new antibodies.
"We had hoped that over time as the pandemic went along, as our fight against this virus went along, we would be expanding our medicine cabinet," Jha told reporters in October. "Because of lack of congressional funding, that medicine cabinet has actually shrunk and that does put vulnerable people at risk."
President Joe Biden told people with compromised immune systems to consult with a doctor.
"New variants may make some existing protections ineffective for the immunocompromised," the president said in October. "Sadly, this means you may be at a special risk this winter. I urge you to consult your doctors on the right steps to protect yourself, take extra precautions."

 Astrong
Astrong 

















.jpg&h=630&w=1200&q=100&v=6e07dc5773&c=1)