Johnson & Johnson beats on earnings, hikes full-year guidance as medtech sales surge
The results come amid investor anxiety over the thousands of lawsuits claiming that J&J's talc-based baby powder and other products caused cancer.
Johnson & Johnson on Thursday reported second-quarter revenue and adjusted earnings that topped Wall Street's expectations, and lifted its full-year guidance as sales from the company's medtech business jumped.
The medtech division provides devices for surgeries, orthopedics and vision. The company is benefitting from a rebound in demand for non-urgent surgeries among older adults, who deferred those procedures during the pandemic.
That increased demand has been observed by health insurers like UnitedHealth Group and Elevance Health.
Here's how J&J results compared with Wall Street expectations, based on a survey of analysts by Refinitiv:
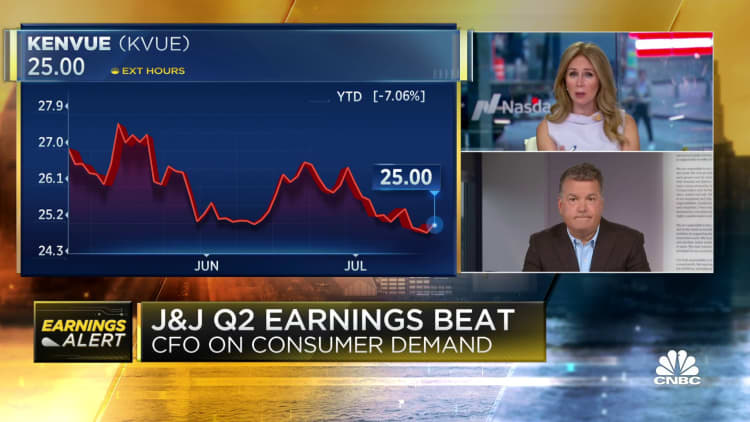
Earnings per share: $2.80 adjusted, vs. $2.62 expectedRevenue: $25.53 billion, vs. $24.62 billion expectedShares of J&J rose nearly 5% in morning trading Thursday. The stock has dropped more than 5% for the year, putting the company's market value at roughly $433 billion.
J&J, whose financial results are considered a bellwether for the broader health sector, said its sales during the quarter grew 6.3% over the same period last year.
The pharmaceutical giant reported a net income of $5.14 billion, or $1.96 per share. That compares with a net income of $4.8 billion, or $1.80 per share, for the same period a year ago.
Excluding certain items, adjusted earnings per share were $2.80 for the period.
J&J is now forecasting full-year sales of $98.80 billion to $99.80 billion, about $1 billion higher than the guidance provided in April.
The company raised its 2023 adjusted earnings outlook to $10.70 to $10.80 per share, from a previous forecast of $10.60 to $10.70 per share.
The full-year guidance includes results from J&J's consumer health business, which spun out as an independent company under the name Kenvue in early May.
J&J owns nearly 90% of Kenvue shares and plans to reduce its stake through an exchange offer that could launch "as early as the coming days," J&J CFO Joseph Wolk said during an earnings call.
That process will allow J&J shareholders to exchange all or a portion of their shares for Kenvue's common stock.
In this photo illustration the stock trading graph of Johnson and Johnson is seen on a smartphone screen.
Rafael Henrique | SOPA Images | LightRocket | Getty Images
Sales for the company's medical devices business rose to $7.79 billion, up 12.9% from the second quarter of 2022.
J&J said growth came from electrophysiological products, which evaluate the heart's electrical system and help doctors understand the cause of abnormal heart rhythms. Wound closure products and devices for orthopedic trauma, or serious injuries of the skeletal or muscular system, also contributed.
J&J said its acquisition of Abiomed, a cardiovascular medical technology company, in December helped fuel that growth.
"These strong results continue to show that our efforts were able to improve the growth of the medtech business are working," J&J CEO Joaquin Duato said during an earnings call.
Wolk added during the call that recently launched medtech products are a "significant factor" driving the higher growth trajectory of the business.
Pharmaceutical business
J&J reported $13.73 billion in pharmaceutical sales, which grew more than 3% year over year. Excluding sales of its unpopular Covid vaccine, the pharmaceutical division raked in $13.45 billion.
The business is focused on developing drugs across different disease areas.
The company said the growth was driven by sales of Darzalex, a biologic for the treatment of multiple myeloma, Erleada, a prostate cancer treatment, and the blockbuster drug Stelara, which is used to treat a number of immune-mediated inflammatory diseases.
J&J will lose patent protection on Stelara later this year.
Growth was partially offset by the decline in sales of arthritis drug Remicade, which faces competition from biosimilars, or lower-cost medicines almost identical in structure.
This quarter was the first without any U.S. sales from J&J's Covid vaccine, which brought in $285 million in international revenue.
In April, the company said it expects no domestic revenue beyond what it reported during the first quarter because its commitments under government contracts are complete.
Duato said J&J's pharmaceutical pipeline is "progressing well."
He highlighted experimental drugs such as Milvexian, an oral treatment that aims to prevent blood clots, that are inching toward potential Food and Drug Administration approval.
Duato said the strong pharmaceutical results and potential upcoming drug launches make J&J "very confident" it can meet the division's 2025 annual sales target of $57 billion.
Kenvue results, talc litigation
J&J said the consumer health business raked in $4.01 billion in sales for the quarter, up 5.4% from the same period a year ago.
That growth primarily came from over-the-counter products such as Tylenol, the pain reliever Motrin and upper respiratory products. Skin health and beauty products under the Neutrogena brand contributed to international sales growth.
Kenvue reported its first quarterly results on Thursday.
J&J's quarterly results come amid investor anxiety over the thousands of lawsuits claiming that the company's talc-based products were contaminated with the carcinogen asbestos, which caused ovarian cancer and several deaths.
Those products, such as J&J's namesake baby powder, now fall under Kenvue. But J&J will assume all talc-related liabilities that arise in the U.S. and Canada.
In April, J&J's subsidiary LTL Management filed for bankruptcy in New Jersey, proposing to pay nearly $9 billion to settle more than 38,000 lawsuits and prevent new cases from coming forward.
It's the company's second attempt to resolve talc claims in bankruptcy court after a federal appeals court rejected an earlier bid.
Most litigation has been halted during the bankruptcy proceedings. But a bankruptcy court allowed a trial in Oakland, California to proceed.
On Tuesday, a jury decided that J&J must pay $18.8 million to a man who said he developed cancer from exposure to its baby powder.
J&J vice president of litigation Erik Haas said during the earnings call that the company plans to appeal the verdict. He called it "irreconcilable with decades of independent scientific evaluations confirming Johnson & Johnson's baby powder is safe, does not contain asbestos and does not cause cancer."
Haas added that J&J will not pay the verdict award while the bankruptcy proceeding continues, and "the decision has absolutely no impact on that process."

 FrankLin
FrankLin