Moderna asks FDA to authorize Covid vaccine for kids under 6 years old
The FDA has promised to move quickly to authorize shots for infants, toddlers and preschoolers once the vaccine makers submit complete applications.

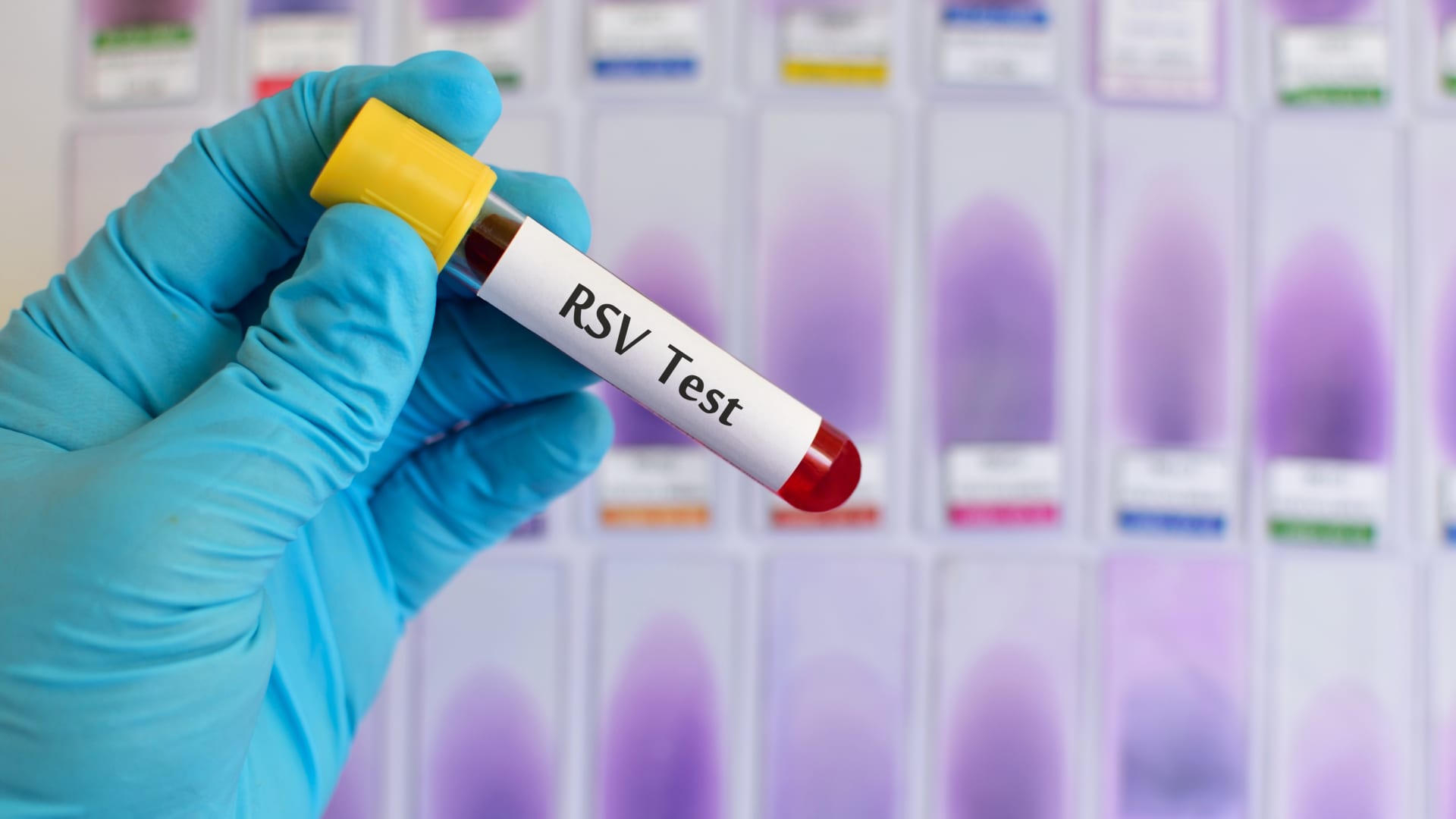
With her husband Stephen by her side Erin Shih hugs her children Avery 6, and Aidan, 11, after they got their second Moderna COVID-19 vaccines at Kaiser Permanente Los Angeles Medical Center on Friday, June 25, 2021.
Sarah Reingewirtz | MediaNews Group | Getty Images
Moderna on Thursday asked the Food and Drug Administration to authorize its Covid vaccine for children ages 6 months to 5 years old.
The vaccine was about 51% effective against infection from the omicron variant in children under 2 years old and about 37% effective among 2- to 5-year-old kids, according to a company press release. Dr. Paul Burton, Moderna's chief medical officer, said those levels are similar to two-dose protection for adults.
The protection Moderna's vaccine provides against infection has declined substantially from the high-water mark of 90% effectiveness when the shots first rolled out. The omicron variant, which has more than 30 mutations, is adept at evading the antibodies that block the virus from invading human cells.
However, Burton said children under 6 years old who receive two doses should have high levels of protection against severe illness. Adults have about 1,000 units of antibody after two shots with at least 70% protection against severe disease, while children in the study had 1,400 to 1,800 units of antibody after two doses, he said.
"What we know is that those levels of antibody will translate into very high protection against severe disease and hospitalization," Burton said. None of the children in the study were hospitalized with Covid, he added.
Moderna plans to study a booster dose for children under age 6 with a redesigned shot that targets omicron as well as the original strain of the virus that emerged in Wuhan, China. One of the reasons vaccine effectiveness against infection has declined so steeply is because the current shots are still targeting the Wuhan strain, even though the virus has evolved dramatically since it was first discovered in late 2019.
If authorized by the FDA, children under age 6 would receive two 25-microgram shots, a much smaller dose than the 100 microgram shots currently approved by the FDA as a primary vaccination series for adults. Burton said the safety profile for kids is reassuring, with 0.2% of the children developing fevers of 103 degrees Fahrenheit, or 40 degrees Celsius. About 17% of kids under 2 years old developed a fever of 100 degrees Fahrenheit while slightly more than 14% of kids from 2 to 6 years old developed such a fever, according to a press release Moderna issued in March on its study results.
Kids under 6 years old are in the only age group in the U.S. that is not yet eligible for vaccination. The FDA has promised to move quickly to authorize shots for infants, toddlers and preschoolers once the vaccine makers submit complete applications.
Dr. Peter Marks, who heads the FDA office responsible for vaccines, told the Senate health committee this week that the drug regulator's committee of independent advisors will meet to fully review the data.
"We will proceed with all due speed once we have complete applications," Marks said. He told the committee that the FDA will publish a timeline in the next week for advisory committee meetings on several emergency-use applications. The FDA is in the process of clearing several potential dates for the committee to meet in June, according to a person familiar with matter.
Parents have been waiting for months for a way to protect their children against the virus. During the winter omicron wave, children younger than 5-years-old were hospitalized with Covid at five times the rate of the pandemic's peak, when delta was dominant, according to the Centers for Disease Control and Prevention. About 75% of children under 11 years old had been infected with Covid as of February, according to data released by the CDC this week.
The FDA had originally sought to fast track authorization of Pfizer's Covid vaccine for children under age 5 in February by clearing the first two doses of the three-shot vaccine. However, Pfizer decided to postpone its application and wait for data on the third shot, because the results from the first two doses weren't good enough.
Pfizer CEO Albert Bourla, in a podcast interview, said the first two shots only had 30% to 40% efficacy, but he expects the third dose to significantly improve protection. The vaccine has a three-microgram dosing level, much smaller than the 30-micrograms used for adults.
Bourla said he hopes Pfizer's vaccine will receive FDA authorization in June.

 Hollif
Hollif