FDA expands monkeypox vaccine authorization to increase dose supply, allows shots for children
The FDA will allow health-care providers to administer the shots through intradermal injection, or between the layers of the skin, for adults.
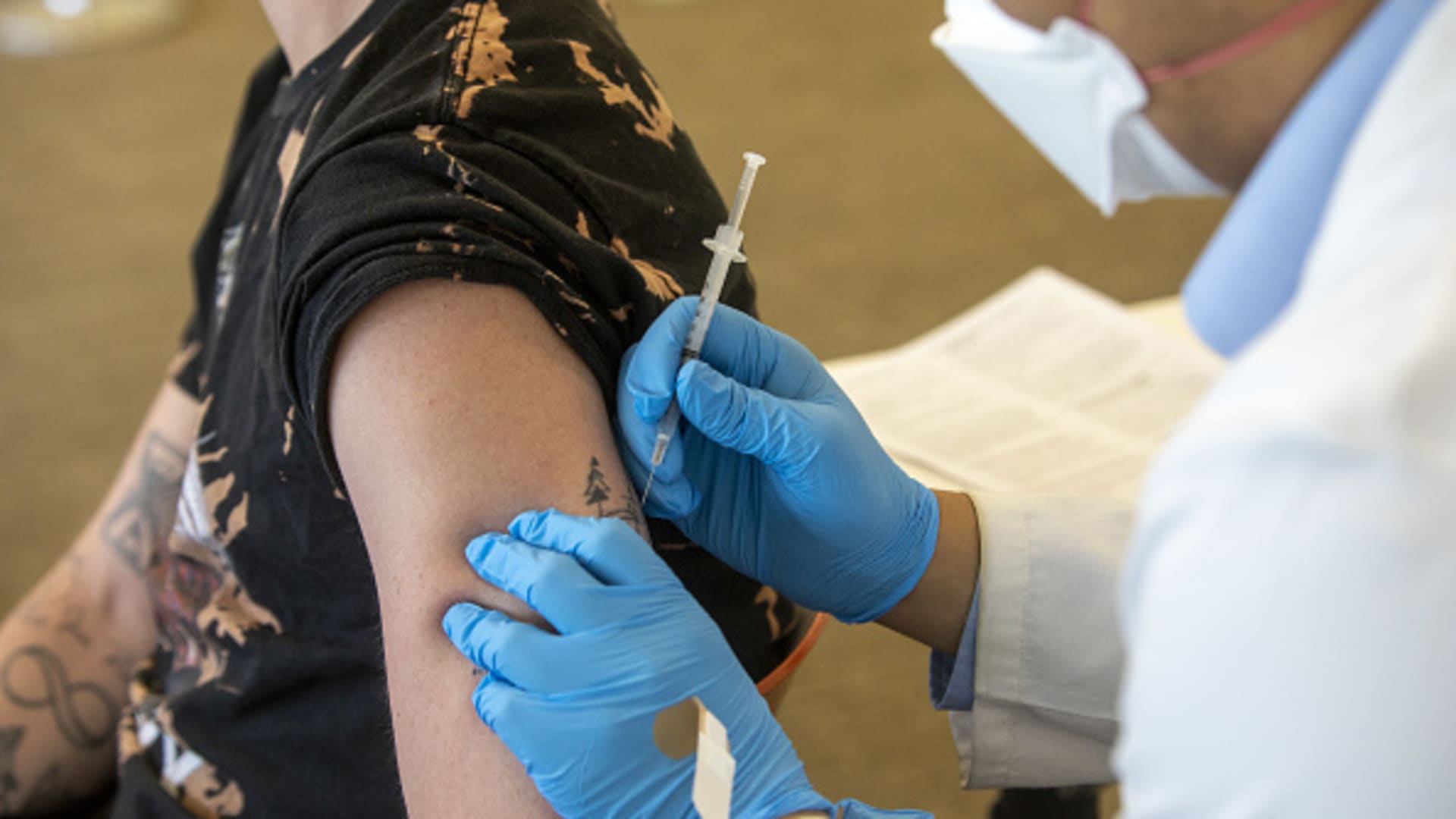
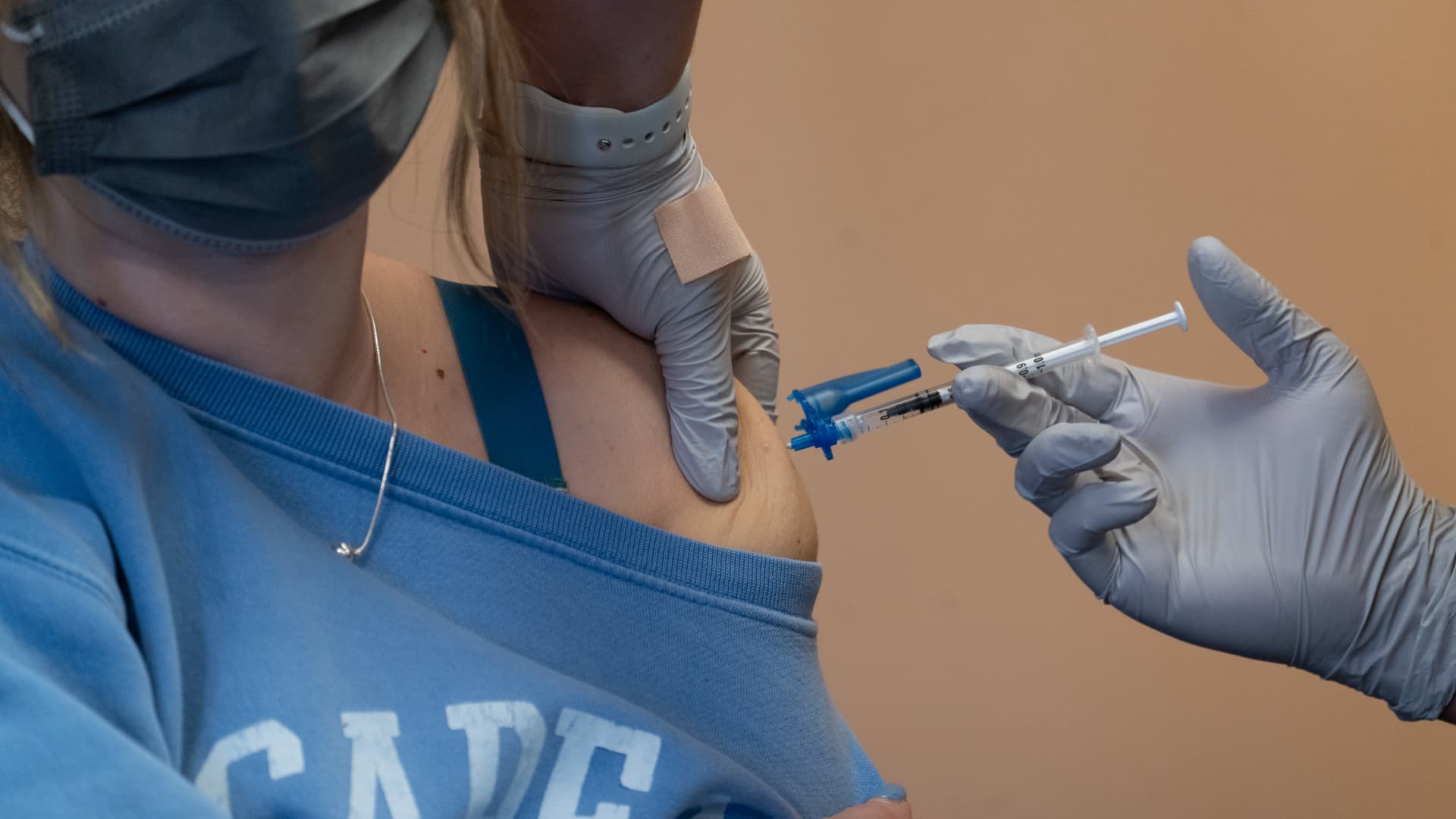
A health worker administers a dose of the Bavarian Nordic A/S Jynneos monkeypox vaccine at a vaccination site in West Hollywood, California, on Wednesday, Aug. 3, 2022.
Jill Connelly | Bloomberg | Getty Images
The Food and Drug Administration on Tuesday expanded its authorization for the monkeypox vaccine in a way that would significantly boost the limited supply of shots.
The FDA is also now allowing children to receive the vaccine if they are at high risk of monkeypox infection. Dr. Peter Marks, head of the FDA's vaccine division, said there has been an increase in possible exposures among children over the past week.
Health-care providers can now administer the shots to adults through intradermal injection, or between the layers of the skin. This will increase the supply of doses by as much as fivefold, according to the FDA. The vaccine is traditionally administered through subcutaneous injection, which goes into the fat layer beneath the skin.
The intradermal injections for adults use a lower volume dosage that will allow 400,000 vials in the strategic national stockpile to provide up to 2 million shots, according to Robert Fenton, the White House monkeypox response coordinator.
Children will receive the vaccine through the typical subcutaneous injection. Marks said there isn't enough data to allow intradermal injections for kids and this method is also difficult to administer to very young children.
Jynneos is the only FDA-approved monkeypox vaccine in the U.S. The shots are administered in two doses 28 days apart. Jynneos is manufactured by Bavarian Nordic, a biotech company based in Denmark.
FDA Commissioner Dr. Robert Califf said there isn't data on how well the vaccine prevents disease because there were no smallpox cases and monkeypox outbreaks have been small in the past. The vaccine was approved by the FDA in 2019 based on immune response data.
Dr. Rochelle Walensky, director of the Centers for Disease Control and Prevention, said the public health agency is launching various studies to collect real-world effectiveness data as the shots roll out across the country.
Demand for the shots has outstripped available supply as the monkeypox outbreak grows. People have struggled to get appointments, which book up quickly, and there have been long lines outside clinics around the country.
The U.S. is fighting the largest monkeypox outbreak in the world with nearly 9,000 cases across 49 states, Washington, D.C., and Puerto Rico, according to the CDC. Health and Human Services Secretary Xavier Becerra declared the outbreak a public health emergency last week.
Monkeypox is rarely fatal, and no deaths have been reported in the U.S. so far. But the virus causes lesions that can be very painful. Some patients need hospitalization to manage the pain.
Monkeypox is primarily spreading through skin-to-skin contact during sex, according to public health officials. But people can also catch the virus through close physical contact in general, such as hugging and kissing, as well as through contaminated materials such as towels or bedsheets.
Gay and bisexual men are at the highest risk of infection right now. About 98% of patients who provided demographic information to clinics identified as men who have sex with men, according to the CDC. But monkeypox could begin to spread more in the broader population if the outbreak is not contained.
Children at a day care center in central Illinois may have been exposed to the virus last week after a caretaker at the facility tested positive. Walensky said infections and exposures among children are still relatively rare. Walensky said public health authorities want to make sure the vaccine is available to kids if they are exposed to the virus.
HHS has made more than 1 million doses available to state and local health departments since May. More than 620,000 doses have been shipped to jurisdictions so far, according to HHS.

 Astrong
Astrong