Novavax confident Covid vaccine will receive FDA authorization in June after delays
If the FDA committee endorses Novavax's shot, the drug regulator is almost certain to rapidly authorize it for use in the U.S.
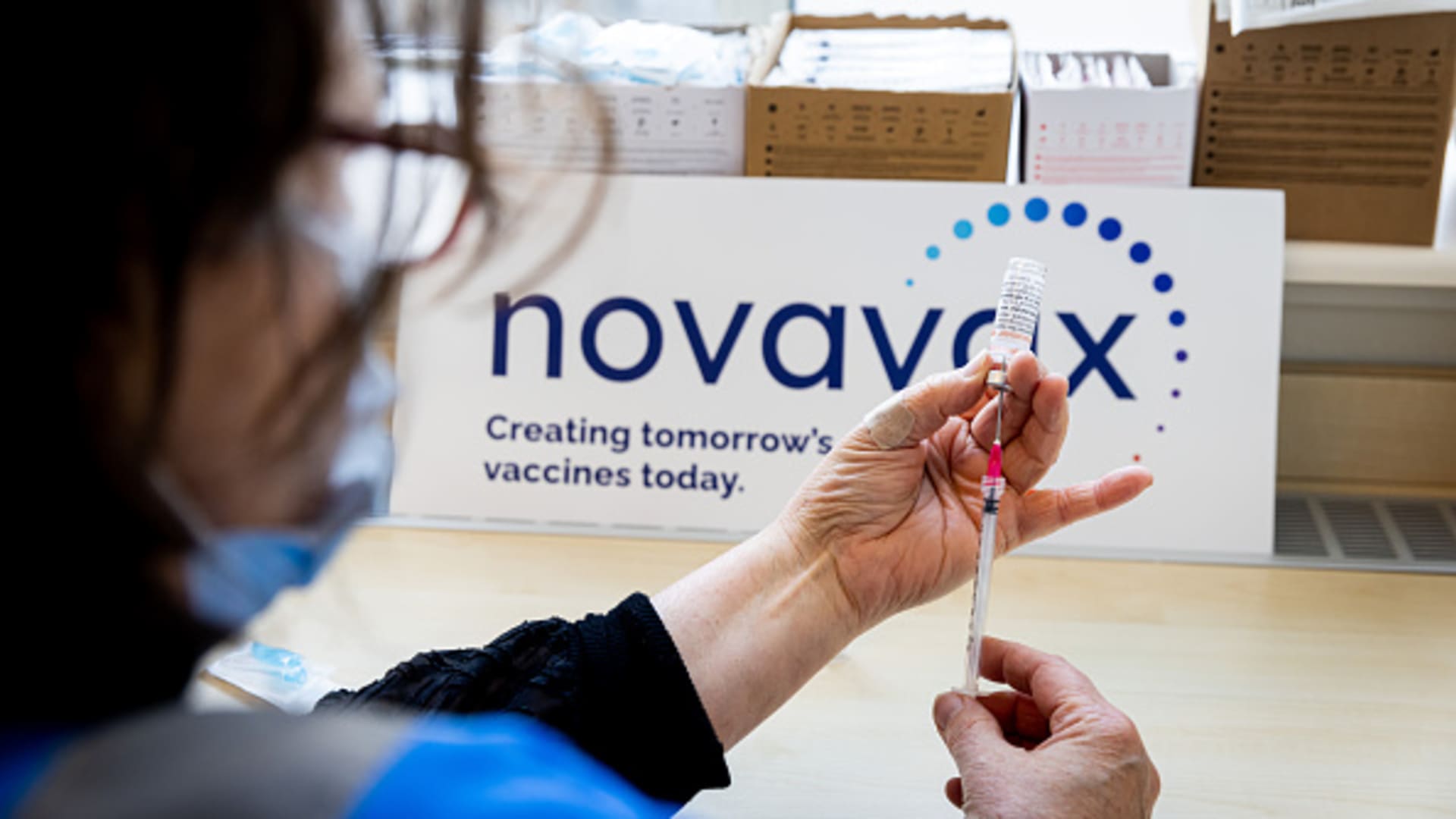
A health worker prepares a dose of the Novavax vaccine as the Dutch Health Service Organization starts with the Novavax vaccination program on March 21, 2022 in The Hague, Netherlands.
Patrick Van Katwijk | Getty Images
Novavax is confident its Covid-19 vaccine will receive the endorsement of the Food and Drug Administration's advisory committee early this summer, executives said this week.
The FDA committee is scheduled to meet on June 7 to review Novavax's submission. An endorsement from the committee, which is made up of independent experts, would mean the drug regulator is almost certain to quickly authorize the two-dose vaccine for use in the U.S.
CEO Stanley Erck said this week that Novavax's manufacturing partner in India, Serum Institute of India, has successfully completed an FDA inspection. Erck told analysts during the company's first-quarter earnings call that he fully expects the committee will authorize the vaccine for adults.
Chief Commercial Officer John Trizzino, in an interview with Bank of America, said all signs point toward a positive recommendation from the committee next month.
"We're fully expecting based upon our submission, based upon all the back and forth questions that have been asked and answered, based upon the inspection at Serum, to come out of that meeting with a recommendation for emergency use authorization," Trizzino said during Bank of America's virtual health-care conference on Wednesday evening.
The FDA has been reviewing Novavax's submission for months. The vaccine maker asked the drug regulator to authorize the vaccine in January, but federal health officials said the application was complex.
"This is an incredibly complex review process that involves review of not just clinical data but also manufacturing data that will be needed to make a determination about emergency use authorization," Dr. Doran Fink, deputy director of clinical review at the FDA's vaccine division, told the Centers for Disease Control and Prevention's committee of independent vaccine advisors last month.
If Novavax's vaccine is authorized by the FDA, it will be first new shot to hit the market in the U.S. in more than a year. Pfizer, Moderna and Johnson & Johnson are the three vaccines currently used in the U.S., and the FDA last week limited the use of J&J's shots.
The vaccine would enter the U.S. market at a time when 76% of adults are already fully vaccinated. Trizzino said on Wednesday that Novavax's shots would offer choice to the remainder of the adult population that would prefer not to receive an mRNA vaccine. Novavax's vaccine uses more conventional protein technology, whereas Pfizer's and Moderna's use messenger RNA platforms first authorized during the coronavirus pandemic. Trizzino said the shots could also play an important role as booster doses and in teenagers ages 12 to 17.
Novavax has submitted its data from teenagers to the FDA and is also filing data on booster doses, Chief Medical Officer Philip Dubovsky said during the company's earnings call. It's unclear, however, when the FDA may consider the company's shots for teens and as booster doses.
FDA authorization of the vaccine would come right as the drug regulator is considering redesigning Covid shots this fall to target mutations the virus has developed over the past two years. All of the current vaccines, including Novavax, target the spike protein of the original strain of the virus that emerged in Wuhan, China, in 2019. As the virus has evolved, the shots have become less effective at blocking infections.
Novavax plans to launch a clinical trial this month on a version of the vaccine that targets omicron mutations, Erck said during the company's earnings call. Trizzino, during the Bank of America interview, said the goal is to have the shots ready by October for a fall vaccination campaign should the FDA decide to move forward with updating the shots.
"Our thinking is in the fall, we need to be ready to do what our customer wants," Trizzino said, referring to the U.S. government. "We intend to have the clinical data, the package that's filed for that and then be able to deploy in the timeframe of October."
It's unclear how many shots the U.S. government would order should the vaccine receive authorization. Erck said Novavax is in discussions now with the U.S. on how the company can support demand. Novavax has received $1.8 billion from the U.S. government under Operation Warp Speed to deliver 100 million doses, though the government will decide how many shots it wants after FDA authorization.
Novavax stock has dropped 13% this week due to uncertain demand for the shots and after the company missed Wall Street's first-quarter earnings and revenue expectations. Although Novavax maintained its 2022 sales guidance of $4 billion to $5 billion, CFO Jim Kelly said the company has not yet received an order from COVAX, the international alliance that procures shots for poorer nations. It's unclear how much COVAX may order, Kelly said, which could put downward pressure on the sales guidance.
Last year, Novavax signed a memorandum of understanding to make 1.1 billion doses of its vaccine available to COVAX, and the company previously said it has the capacity to manufacture 2 billion doses in 2022. However, Novavax's vaccine rollout around the world has gotten off to a sluggish start this year.
Novavax delivered 42 million doses in the first quarter to markets where the vaccine is already authorized, including the European Union, Canada, South Korea, Australia, New Zealand and Indonesia. However, the company expects shipments and revenue to increase in the second quarter as its fulfills an order of 42 million doses from the EU, Trizzino told analysts during the earnings call.
Novavax's vaccine uses different technology than Pfizer's and Moderna's shots. The Pfizer and Moderna vaccines deliver mRNA to the body's cells, which then produce harmless copies of the virus spike protein, which induces an immune response that fights Covid. The spike protein is the tool the virus uses to invade human cells.
Novavax's fully synthesizes the copies of the spike protein outside the human body. The company inserts the genetic code for spike in a baculovirus which then infects cells for a certain type of moth. Novavax then harvests the spike from those cells and purifies them for the shot. The vaccine also uses what's known as adjuvant, purified from the bark of a South American tree, to boost the immune response.
Novavax's U.S. and Mexico clinical trial found that its vaccine was 90% effective at preventing mild illness and 100% effective at preventing severe illness. However, the trial was conducted well before the omicron variant emerged, which has undermined vaccine effectiveness against infection.
Novavax released results from a lab study in December which found that its vaccine still triggered an immune response against omicron. The study found that a third boosted the immune response to levels similar to the U.S. and Mexico clinical trial, suggesting a high level of protection with a third shot.

 ValVades
ValVades 




















.jpg&h=630&w=1200&q=100&v=6e07dc5773&c=1)




.jpg&h=630&w=1200&q=100&v=6e07dc5773&c=1)