Dr. Mohammad Miqdady Presents UAE Pediatric Crofelemer Investigator – Initiated Trial Results at NASPGHAN 2025
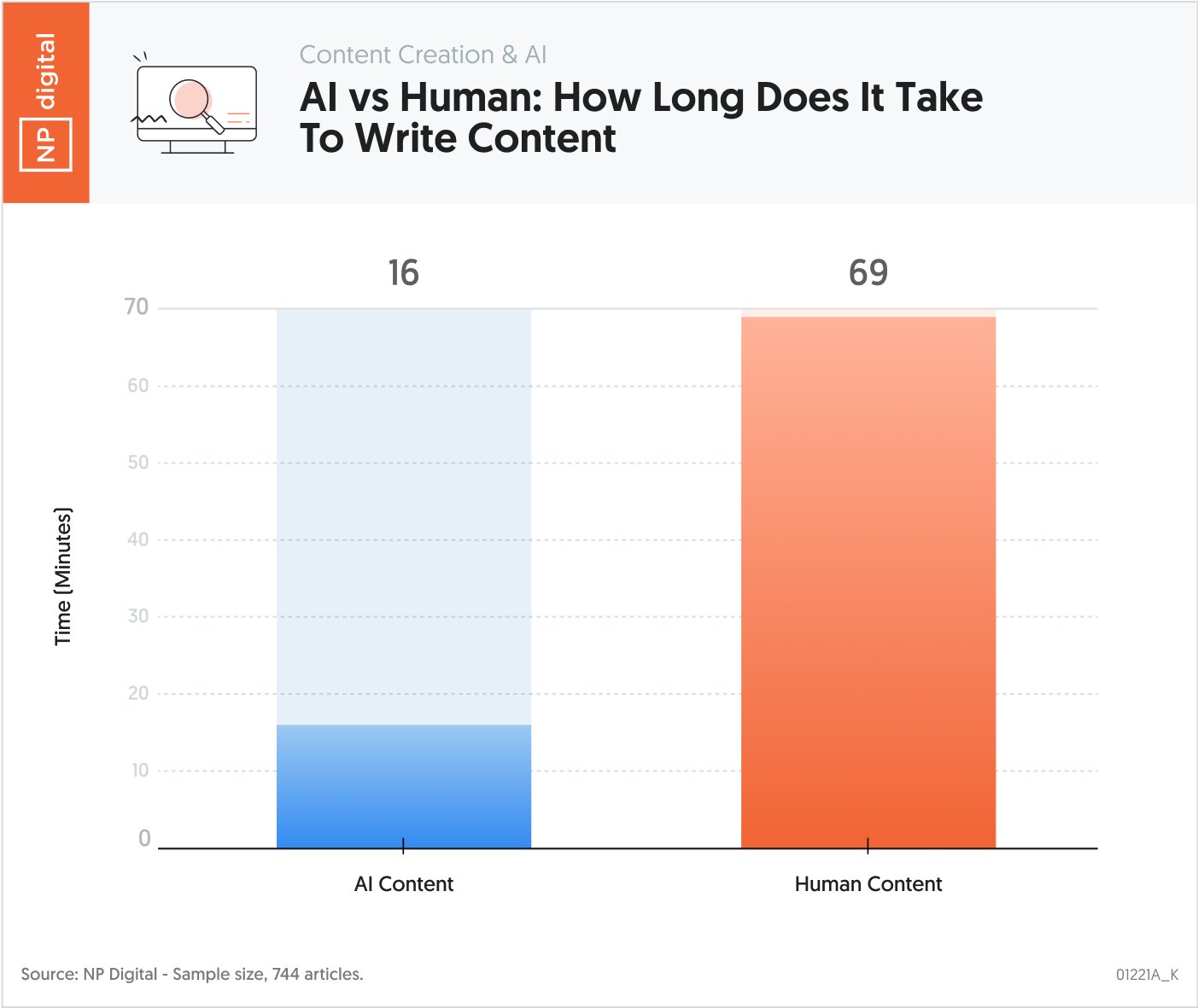
The importance of Total Parenteral Nutrition (TPN) is significant as it has a long lethal natural history. Crofelemer can potentially extend and save the lives of microvillus inclusion disease patients, reducing the volume of the total parenteral support (PS)...

The importance of Total Parenteral Nutrition (TPN) is significant as it has a long lethal natural history. Crofelemer can potentially extend and save the lives of microvillus inclusion disease patients, reducing the volume of the total parenteral support (PS) necessary for them to survive. Groundbreaking PS reduction of up to 37% is unprecedented; No approved treatments exist for MVID.
Patients with intestinal failure due to short bowel syndrome are unable to absorb the fluids, electrolytes, and nutrients required to survive and thrive. Intestinal failure is a debilitating, lifelong condition that often requires patients to receive life-sustaining fluids, electrolytes, and nutrients through intravenous administration. This consists of total parenteral nutrition (TPN) with supplemental intravenous fluids, which together constitute parenteral support (PS).
“Most intestinal failure patients require PS up to 7 days a week, and sometimes for 20 hours or more per day,” said Lisa Conte, Jaguar Health’s founder, president, and CEO.
Results from an ongoing UAE clinical trial evaluating crofelemer in pediatric intestinal failure patients were unveiled on November 8, 2025, at the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Annual Meeting in Chicago, offering early evidence that the therapy may reduce reliance on parenteral nutrition and improve gastrointestinal outcomes in children with severe intestinal malabsorption.
The data, presented by Dr. Mohammad Miqdady, Division Chief of Pediatric Gastroenterologist, Hepatology & Nutrition Division at Sheikh Khalifa Medical City, Abu Dhabi, reflects interim findings from an open-label, single-arm, non-randomized 12-week dose-escalation study assessing the safety and effectiveness of crofelemer in pediatric patients with intestinal failure (IF), a complex condition commonly caused by congenital intestinal disorders or short bowel syndrome (SBS) and often requiring long-term intravenous nutritional support.

“This patient population has historically had limited therapeutic options beyond lifelong parenteral nutrition, which itself carries significant risks despite being life-sustaining,” Dr. Miqdady said. “Our objective was to determine whether crofelemer could be administered safely in children and whether it could reduce dependence on parenteral support while improving stool consistency and hydration.” The results of the trial support this objective, and the trial is continuing.
Crofelemer is an oral antisecretory therapy that targets intestinal chloride ion channels, including the cystic fibrosis transmembrane conductance regulator (CFTR) and calcium-activated chloride channel (CaCC), mechanisms directly associated with intestinal fluid loss and diarrhea. The drug is already FDA-approved for specific adult gastrointestinal uses, but this study represents an important step in understanding its potential application in pediatric intestinal failure.
In the UAE trial, children between the ages of 1 and 18 years, with a requirement for parenteral support for more than 60 days, were enrolled. Patients with recent bowel surgery, active infection, malignancy, or immunosuppressant use were excluded. Eligible participants received oral crofelemer in increasing doses, 3 mg/kg three times daily for two weeks, 6 mg/kg three times daily for two weeks, and 9 mg/kg three times daily for the final eight-week period. Throughout the 16-week observation window, outcomes were tracked through clinical exams, laboratory data, nutritional logs, and caregiver-recorded daily diaries monitoring stool frequency, hydration, urine output, and parenteral support volume.
“Safety was our first priority,” Dr. Miqdady emphasized. “What is particularly encouraging is that even at the highest dose of 9 mg/kg, administered three times daily, no clinically significant adverse effects were observed.”
“We also saw a trend toward improved hydration, reflected by increased urine output, which is significant in managing overall fluid homeostasis,” Dr. Miqdady noted.
“These findings represent early progress, but they matter because they address a fundamental unmet need,” Dr. Miqdady said. “Reducing the number of hours a child must depend on intravenous nutrition is not just a clinical milestone; it affects infection risk, vascular access preservation, caregiver burden, quality of life, and nutritional autonomy.”
Pediatric intestinal failure remains a high-complexity condition with limited approved therapies. Current management focuses heavily on nutritional support, surgical reconstruction when feasible, and infection prevention. The possibility of an oral therapy that could lessen parenteral support requirements, without introducing new safety concerns, raises meaningful clinical implications for a patient population where even incremental improvements can translate into long-term benefits.
“These are early results, but they reinforce the importance of continued evaluation,” Dr. Miqdady said. “Crofelemer is not being positioned as a replacement for comprehensive intestinal care, but if it can safely support reductions in parenteral nutrition needs while improving gastrointestinal stability, that is a meaningful advancement worth pursuing.”
Further data from the full patient cohort, including longer-term outcomes and expanded clinical measures, are expected to be shared in subsequent presentations as the study progresses.

 Konoly
Konoly